
Introduction to General, Organic and Biochemistry
11th Edition
ISBN: 9781285869759
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
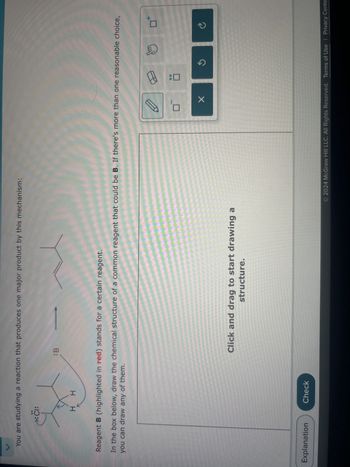
Transcribed Image Text:You are studying a reaction that produces one major product by this mechanism:
CI:
H H
:B
Reagent B (highlighted in red) stands for a certain reagent.
In the box below, draw the chemical structure of a common reagent that could be B. If there's more than one reasonable choice,
you can draw any of them.
Explanation
Check
Click and drag to start drawing a
structure.
田
X
$
C
टे
+
© 2024 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Cente
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- More than one reagent/reaction will be necessary to produce the product from the reactant provided. You must provide all necessary reaction conditions (HBr, HgSO4, etc.) and the products produced after each step of the synthesis.arrow_forwardoarrow_forwardDraw the organic molecule(s) which is(are) formed in the following reaction. Do not include molecules like H₂O or HCl. (You have 2 chances until the answer will be given; you have already tried 0 times) H₂ CH CH₂ HC HC H C CH ***** CH Br + Compound #1 H₂C H₂ C. Draw only one molecule in each drawing box. NHarrow_forward
- Bhhhhhhharrow_forwardAt the beginning of a reaction, there is virtually no product. O True O Falsearrow_forwardA glycerol molecule and three butyric acid molecules are shown. Delete atoms and add bonds as necessary to form a triacylglycerol (triglyceride). Draw only the triacylglycerol product. Select Draw Rings More Erase / |||||| COH G H H н-с H Ć H O H H H I O —1 O HO ||| C H₂ UI H₂ с H₂ с H₂ CH CH3 CH₂ Q2 Qarrow_forward
- Fill in Intermediate & Product ОН ток ·CH ₂ I Parrow_forwardDraw the organic molecule(s) which is(are) formed in the following reaction. Do not include molecules like H,0 or HCI. (You have 3 chances until the answer will be given; you have already tried 0 times) H, H.C CH, + H,C ČH, CH, Draw only one molecule in each drawing box. If need more compounds for your answer, click this button ADD DRAWING BOX Compound #1arrow_forwardQ2. Carbonic Anhydrase or carbonic dehydratase is a family of metalloenzymes containing zinc (Zn) ion in its active site. Carbonic anhydrase can greatly increase the rate of this reaction, reaching a reaction rate of 104 - 106 per second. It is defined as the enzyme found in red blood cells, other parts of animals and plants, that breaks down carbonic acid with carbon dioxide and water. CO:(2) – H»O(1) – H.CO:(ag) For the carbonic anhydrase activity specified by the equation above, evaluate the standard reaction Gibbs energy at 25 °C?arrow_forward
- Draw the organic molecule(s) which is(are) formed in the following reaction. Do not include molecules like H,0 or HCI. (You have 3 chances until the answer will be given; you have already tried 0 times) HC NH, HC. CH, Draw only one molecule in each drawing box. If need more compounds for your answer, click this button ADD DRAWING BOX Compound #1arrow_forwardIndique si las siguientes reacciones presentadas siguen el mecanismo SN1 o SN2. 1 H3C. CH,01 CH,OH CH CH,COO1 H3C Br CH,COOH CH CH, H,O H,C CH,CH,OH CI O a. 1-SN1, 2-SN2, 3-SN1 Ob.1-SN1, 2-SN1, 3-SN1 Oc1-SN2, 2-SN1, 3-SN1 Od.1-SN2, 2-SN1, 3-SN2 2.arrow_forwardC5H8 + 7O2 → 5CO2 + 4H2O type of reactionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning