
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Determine the product of the reaction
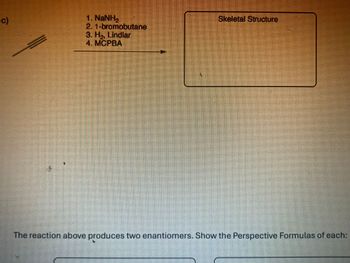
Transcribed Image Text:c)
1. NaNH
2. 1-bromobutane
3. H₂, Lindlar
4. MCPBA
Skeletal Structure
The reaction above produces two enantiomers. Show the Perspective Formulas of each
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Complete the following zig-zag structure so that it represents (2R,3S)-3-chlorobutan-2-ol. Please explain you answer.arrow_forward5) relationship between the pairs of structures. NOTE: Each term may be used more than Choose the term from the five terms listed below that BEST describes the once and not all terms need be used. Identical Diastereomers Enantiomers Constitutional isomers Not isomers CH3 CH3 ÇI H3C-Br CH3 Br -CI H,C. D-H H3C- Br H- -D Br CH3 -CI ČH3 OH H3C, CHO OHC, OH OH H. HO CHO OHC CH3 H. OH ÓHarrow_forwardPlease help with the following... Draw the bond line structures for the reactants and the products obtained in the following reactions: 1. m-chlorocumene +NaNiPr2, HNiPr2 2. 4-tertbutyl-3-methyl anisole + HNO3, H2SO4 3. o-isopropyl acetophenone + PhCH2COCl, AlCl3arrow_forward
- What are the chemical structure of me сиз сиз (excess] following musing compocents Brz. mono substituted MAHAA 2 G3 77 excess KM 12 छाट reduction E2 Reduction (mild pond) NaBH3 CH 3 Mg Br HLOH H E3 HOW Hyle. bor. Holt aid. H3 oxymer/ demo. H2 Three compounds A, B, and C all have the formula C6H10. All three compounds rapidly decolorize bromine in CC14; all three are soluble in cold concentrated sulfuric acid. Compound A has an absorption in its IR spectrum at about 3300 cm, but compounds B and C do not. Compounds A and B both yield hexane when they are treated with excess hydrogen in the presence of a platinum catalyst. Under these conditions C absorbs only one molar equivalent of hydrogen and gives a product with the formula C6H12. When A is oxidized with hot basic potassium permanganate and the resulting solution acidified, the only organic product that can be isolated is CH3(CH2) 3CO₂H. Similar oxidation of B gives only CH3CH2CO₂H, and similar treatment of C gives only HO₂C(CH2)…arrow_forwardI need an explanation on this problem.arrow_forwardOnly typed solutionarrow_forward
- A2.arrow_forward3. Determine the relationship for the following pairs of structures. Use "E" for enantiomers, "D" for diastereomers, "C" for constitutional isomers, "S" for same, and "NS" (not same) for different compounds. a. b. с. d. е. f. Н н iPr "Де Br Br OH CH3 CH3 Ή OH OH OH Br Br CH3 CH3 Br -Н НО -Н H3C -ОН COOH Br Br Br Н H3C- Br OH CH3 Н CH3 iPr CH3 Н- -Br НО _H H OH OH CH3 ОН -OH COOHarrow_forward! ( plz explain in detail)arrow_forward
- 6. For the following compound, (1) draw the complete Lewis structure; (2) site down the C3- C4 bond axis, and draw the seven Newman projections (0-360 degrees of rotation); (3) label each structure as to being eclipsed (E) or staggered (S). -CEN エ Ctarrow_forwardUse flat representation of rings, not chair in the drawing. Determine the most and least stable. Consider the most stable chair for each of these isomers, and then draw the most stable and least stable isomer based on a comparison of the best chair for each one.arrow_forwardPlease don't provide handwritten solution .....arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning