
World of Chemistry, 3rd edition
3rd Edition
ISBN: 9781133109655
Author: Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher: Brooks / Cole / Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
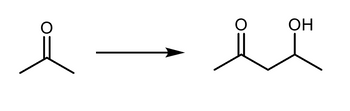
what is the synthesis to get to the product?
Transcribed Image Text:HO
- iN
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- f. + FO 0:arrow_forward1-86 The specific heats of some elements at 25oC are as follows: aluminum = 0.215 cal/g · oC; carbon (graphite) = 0.170 caI/g oC; iron = 0.107 cal/g mercury = 0.033 1 caI/g oC. (a) Which element would require the smallest amount of heat to raise the temperature of 100 g of the element by 10oC? (b) If the same amount of heat needed to raise the temperature of 1 g of aluminum by 25oC were applied to 1 g of mercury, by how many degrees would its temperature be raised? (c) If a certain amount of heat is used to raise the temperature of 1.6 g of iron by 10oC, the temperature of 1 g of which element would also be raised by 10oC, using the same amount of heat?arrow_forward← X g 單 0 Priva Tern C My Account - MySam - Sam Hou X Content X Q mathway calculator - Search https://shsu.blackboard.com/ultra/organizations/_202946_1/cl/outline LI Lets start with the medical components. We need to determine how many units of the syringe connections can be made out of one metric ton of PEI, or around 2205 lbs of the plastic. X M Mathway | Basic Math Problem S X + 1 metric ton = 2205 lbs Starting material - PEI 1 metric ton ≈ 2205 lbs Calculation for number of units of each component: total weight of starting material PEI X Products Medical components Stopcock with Luer Connection, 1-way, male lock unit weight 2.72 g = 3/500 lbs 1 unit of medical component 1 weight of unit of component This calculation assumes all starting material is used during the production of the components. Medical components Products Stopcock with Luer Connection, 1-way, male lock unit weight 2.72 g 3/500 lbs Female Luer Lock 3/16" unit weight 0.63 g 7/5000 lbs Units = If necessary round to the…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

World of Chemistry
Chemistry
ISBN:9780618562763
Author:Steven S. Zumdahl
Publisher:Houghton Mifflin College Div