
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
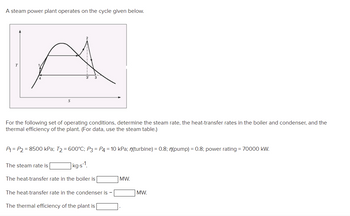
Transcribed Image Text:A steam power plant operates on the cycle given below.
S
For the following set of operating conditions, determine the steam rate, the heat-transfer rates in the boiler and condenser, and the
thermal efficiency of the plant. (For data, use the steam table.)
P₁ = P2 = 8500 kPa; T₂ = 600°C; P3 P4 = 10 kPa; n(turbine) = 0.8; n(pump) = 0.8; power rating = 70000 kW.
kg-s-1
The steam rate is
The heat-transfer rate in the boiler is
The heat-transfer rate in the condenser is -
The thermal efficiency of the plant is
MW.
MW.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
why does P1 = P2 and P3 = P4?
Solution
by Bartleby Expert
Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
why does P1 = P2 and P3 = P4?
Solution
by Bartleby Expert
Knowledge Booster
Similar questions
- As as chemical engineer analyse how does the choice of humidification method, such as adiabatic or isothermal humidification, affect the efficiency and energy consumption of a cooling tower in a chemical process plant?arrow_forwardSteam flowing at a rate of 10 kg/h enters a steam turbine at a velocity of 50 m/s and leaves at a point 5 m below the inlet at a velocity of 300 m/s. The heat loss from the turbine is estimated to be 10 kW, and the turbine delivers shaft work at a rate of 70 kW. Calculate the change in enthalpy transport rate of the process.arrow_forwardadiabatic turbinearrow_forward
- A horizontal steam pipe (60 mm. OD) carries high pressure steam at 230℃ . Wind flows past this pipe at 1 m/s velocity. The ambient temperature is 32℃. Which mode(s) of convection should we consider here? Calculate the total rate of heat loss per m. length of the pipe.arrow_forwardWhat two pressures control the operation of an automatic expansion valve?arrow_forwardQUESTION 8 Which type of TEMA class heat exchanger used for heaters used in canteens ( hotels)? O A. Class C O B. Class R OC. Class A O D. Class B QUESTION 9 A designer reported 5 tube passes for a shell and tube heat exchanger. True False O E e TIONL40arrow_forward
- J 5 A still which produces ethanol from corn mash. Edgar wants to condense the ethanol by running it though a copper tube. If the copper tube can transfer 0.6 btu per second and Edgar wants to produce 10 fifths of ethanol per hour, how long should the tube be?arrow_forwardEnergy is generated uniformly in a 20cm thick wall the steady state temperature distribution of the wall is indicated on the table below; z (cm) T(C) 0 2 4 6 8 10 12 14 16 18 20 150 177 205 230 250 272 292 310 330 345 360 If thermal conductivity of the wall is 15 W/m.K and the indicated temperatures are in °C, determine the average rate of heat generation in unit volume.arrow_forward1. Consider a 1000 MW power plant located in a rural area with 15 ton/day SO2 emissions from a 100 m high stack. The velocity and temperature of the stack gases lead to an effective stack height of 50 m above the physical stack. Estimate the ground level concentration as a function of distance downwind under the following conditions. The emissions are into a clear daytime atmosphere with wind (at 10 m) of 5 m/s. b. The emissions are into a clear nighttime atmosphere with wind (at 10 m) of 2 m/s. The conditions of a. except there is a strong elevated inversion at an altitude of 200 m. a. С.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The