
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
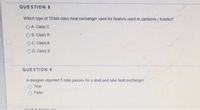
Transcribed Image Text:QUESTION 8
Which type of TEMA class heat exchanger used for heaters used in canteens ( hotels)?
O A. Class C
O B. Class R
OC. Class A
O D. Class B
QUESTION 9
A designer reported 5 tube passes for a shell and tube heat exchanger.
True
False
O E e TIONL40
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Consider how the Beer Heater impacts multiple aspects of distillation columns used in the bourbon industry. In these continuous distillation columns, name three three parameters that are coupled with each other?arrow_forwardDraw the thermal resistance circuit and evaluate the total resistance for the following a. Rectangular object [2 MARKS] Insulation Material. 1 with ki Material.2 with k2 O k, Material.3 with k3 A. T 4=L2 b. Cylindrical pipe, consists of three materials (with length L) [2 MARKS] KI Material. 1 with ki 12 Material.2 with k2 Tei, hị Material.3 with k3 Tw2, hạ K2 K3arrow_forwardAs as chemical engineer analyse how does the choice of humidification method, such as adiabatic or isothermal humidification, affect the efficiency and energy consumption of a cooling tower in a chemical process plant?arrow_forward
- 3 A continuous countercurrent adiabatic rotary drier is being designed for the production of 500 lb/h of a product containing 2% moisture from wet crystal containing 30% moisture, wet basis. The air entering the drier will have a dry-bulb temperature of 230°F and a wet-bulb temperature of 102°F. The air leaving the drier will be at a temperature of 115°F. Because of the small size of the crystals, the highest allowable air velocity is 10 lb dry air/ft2-min of drier cross section. Find: a. Dry air required (lb/min) b. Cross-sectional area of the drier c. Length of drier required if the volumetric coefficient of heat transfer is 25 BTU/ft3-h-°Farrow_forwardInsulating material is used to reduce heat loss from the heating furnace walls to the room. The surface temperature of the insulating material is 100 ° C and the other surfaces 25 ° C. Allowable heat loss up to 160 W / m2 from the wall. If the thermal conductivity of the insulation material is 0.05 W / (m ° C), calculate the required thickness of insulation. insulation thickness = Answer cmarrow_forwardPlease no hand writing solution for other wise down votearrow_forward
- A tire is inflated to a gage pressure of 35 psi at a temperature of 0ºF. Calculate the maximum temperature to which the tire may be heated without the gage pressure exceeding 50 psi. A. 110ºF B. 124ºF C. 139ºF D. 158ºFarrow_forward.240mm steam main pipe, 210m long is covered with 50mm of high temperature insulation (k-0.092 W/m °C) and 40mm of low temperature insulation (k-0.062 W/m °C). The inner and the outer surface temperature as measured are 390 °C and 40 °C respectively. Calculate: 1- The total heat loss per hour. 2- The temperature between two layers of insulation.arrow_forwardCan someone help me identify possible sources of errors while experimenting shell and tube heat exchangers? Explain clearly what will be the cause of this error. Thank you so much!arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The