
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
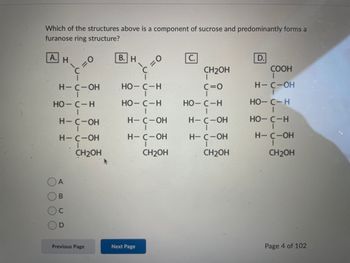
Transcribed Image Text:Which of the structures above is a component of sucrose and predominantly forms a
furanose ring structure?
А.
C
C
н
C
H-C-ОН но-с-н
НО-С-н
НО-С-Н
=0
н-с-он
H-C-OH
CH2OH
A
В
С
D
В. Н
Previous Page
=0
H-
4- с-он
H— C -ОН
CH₂OH
Next Page
CH2OH
c=0
НО-С-н
H— C -ОН
Н— C -ОН
CH2OH
D.
COOH
I
Н-С-ОН
НО-С-Н
но-с-н
H— С-ОН
CH₂OH
Page 4 of 102
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- Part C The L isomer of H-C-OH H-C-OH H-C-OH О CHO О О О CH₂OH OH OH OH OH OH Submit OH ОН OH O OH OH O. OH OH OH O OH OH OH OH OH OH OH Request Answerarrow_forwardWhich of the structures above is a component of sucrose and predominantly forms a furanose ring structure? A. H C I H-C-OH НО-С-Н I Н-С-ОН I H-C-OH I CH2OH A В D B. H =O но-с-н I но-с-н I н-с-он Н-С-ОН I CH₂OH CH2OH I c=0 I но-с-н I H— C -ОН H— C-OH CH₂OH D. COOH H-C-OH НО-С-Н I но-с-н I н-с-он CH₂OHarrow_forwardplease help question 1, a-d Correctly please.arrow_forward
- Draw the first step of lipid hydrolysis using water as a nucleophilearrow_forwardE Untit G pena G tylen G cyan b AnswM (no s Gmon EUX Sora Day Day ORBO6tBah5vDYVv95MSSaAqz8vjye5r9kgU/edit 目 Add-ons Help Last edit was 2 minutes ago ... BIUA 5田回 Arial 12 6. 4. 5 1 2 Observe the number of hydroxyl groups on cellulose, starch, and glycogen. What can the number of -OH bonds tell you about the molecule and its relationship with water? idsarrow_forwardAldose monosaccharides can be oxidized when treated with a mild oxidizing agent. In this reaction, the aldehyde group of the open-chain aldose is oxidized to a carboxylic acid group. In basic solution, ketose monosaccharides can also be oxidized, forming carboxylic acids because they can undergo a rearrangement to an aldose form. Monosaccharides (cyclic hemiacetals) can also react with an alcohol to form acetals, called glycosides, and water. In this reaction, the OH of the anomeric carbon is replaced by the OR group of the alcohol. D-ribose (Figure 1) is treated with a mild oxidizing agent. Edit the structure for D-ribose to show the product of this reaction. M Edit the molecule on the canvas by choosing buttons from the Tools (for bonds), Atoms, and Advanced Template toolbars. The single bond is active by default. Include all hydrogen atoms. ► View Available Hint(s) [1] A H 12D H H H H 7 L EXP. CONT.i L 1 -O-H O H ew -O-H enodic Table -O-H H C N O S CI Br I P Farrow_forward
- Is Raffinose tri-saccharide a reducing sugar? Explain the chemistry of the reducing sugar test and apply it to the tri-saccharide attached.arrow_forward1- Please refer to the Fisher representation of the 5 monosaccharides below: ÇH2OH CHO CHO CHO CHO H- H- он H- OH OH HO HO но- H- OH HO Но- H- -OH H- H- -OH HO- OH HO H- -OH H- -OH HO HO H- OH ČH2OH ČH2CH ČH2CH ČH2CH ČH2CH D-Fructose D-Glucose L-Glucose L-Idose D-Galactose 1a- Which one(s) of the above molecules belong(s) to the subcategory of aldohexoses? and which one(s) of these molecules belong(s) to the subcategory of ketohexoses? Briefly explain your response. 1b- Which one of the above molecules is an enantiomer of D-Glucose? and which one is an epimer of L-Ildose? Briefly explain your response.arrow_forwardIs this saponifiable or not what is this lipidarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON