
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
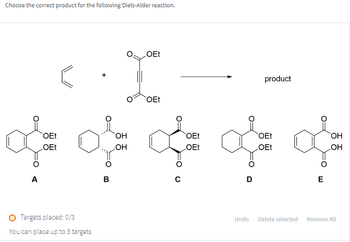
Transcribed Image Text:Choose the correct product for the following Diels-Alder reaction.
OEt
OEt
product
OEt
OEt
OEt
OEt
OEt
OH
OH
of of off of
A
B
C
OEt
D
E
O Targets placed: 0/3
You can place up to 3 targets
Undo
Delete selected
Remove All
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- You run 5 standard proteins listed below on a size-exclusion (gel filtration) column with limit of 200,000Da. Please draw a chromatogram on a separate page with each peak and both axes labeled. Protein B-Amylase Alcohol dehydrogenase Bovine albumin Carbonic anhydrase Cytochome A280 MW 223,800 0.5 82,000 0.6 66,463 0.45 30,000 0.43 12,000 0.8arrow_forwardThe lasonolides are novel polyketides that have displayed remarkable biological activity in vitro against a variety of cancer cell lines. OEt No OH NaBHy OEt OEt OEt OH OH SiMe2Bn R₂SiO R₂SiO Not OEt Li Al4 BnMa Si BnMe₂Si Wittig OH OH OH SiMe2Bn MnO2 OEt OTIPS OTIPS OH OH Part A OTBS + Part B HO OH OH HO OH (-)-lasonolide A NaOH SOCI21 NaOH OH OEt OH HO OH OH OHarrow_forwardA student is attempting to add asparagine to a methionine that is connected to a solid polystyrene bead. They mix together the two components below with DCC, and some of the indicated product is generated. However, more happens in this mixture, resulting in multiple products. What reactivity is happening, and how can it be avoided (to give the indicated product in high yield)?arrow_forward
- You resuspend 1 ml of of a purified chlorophyll sample in 10 ml of 80% acetone, and determine that the optical density of the solution (at 652 nm) is 0.88. What is the concentration of your original preparation?arrow_forwardSelect the product for the following cyclization CHO OH OH OH H OH OH Но HO HO OH но Но - OH HO HO Но. OH H- OH OH H H H ČH2OH 1 3 О 1&2 1 2arrow_forwardMost of the following conclusions regarding the photo shown above are correct Except: All of the following are correct regarding the disk diffusion test photos EXCEPT : Zone of inhibition Chlorine Chlorine Chlorine O-phenylphenol Hexachlorophene O-phenylphenol Hexachlorophene Hexachlorophene O-phenylpheno Quat Quat Quat Staphylococcus aureus (gram-positive) Escherichia coli Pseudomonas aeruginosa (gram-negative) (gram-negative) Select one: O to. Gram negative bacteria are the most sensitive to chemical agents O b. Only one of the four chemicals affected Pseudomonas O c. Gram positive bacteria are the most sensitive to disinfectants O d. Hexachlorophene was effective only against Gram positive bacteria. O and. Chlorine was effective against all bacteria bacteria testedarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON