What product(s) are expected when each of the following compounds reacts with one equivalent of NBS in CCI, in the preser. of light and peroxides? Explain your answers. (a) cyclohexene (b) 3,3-dimethylcyclohexene (c) trans-2-pentene (d) 4-tert-butyltoluene (e) 1-isopropyl-4-nitrobenzene
6

Since you have posted a question with multi-subparts, we will solve first three subparts for you. To get remaining sub-parts solved please repost the complete and mention the sub-parts to be solved.
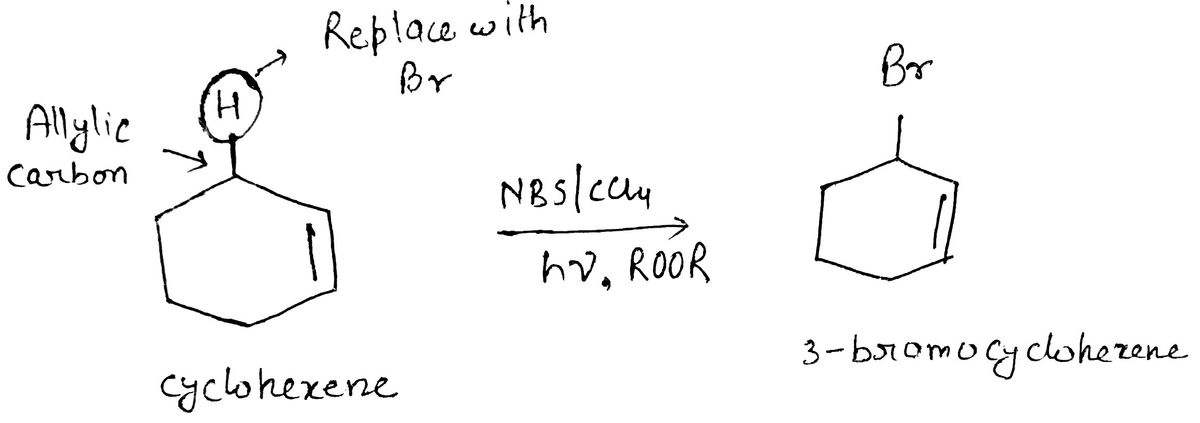
N-bromosuccinamide (NBS)/CCl4 in the presence of light and peroxide is a reagent for bromination, here ROOR is used as peroxide where R is an any alkyl or aryl group. It is highly selective for allylic and benzylic position and this reaction goes via free radical mechanism. In the first step N-succinamide radical and bromine radical has been formed then N-succinamide radical abstract allylic or benzylic hydrogen and form a new radical which is called initiation step. In second step, new radical shows conjugation with double bond and then bromine radical form bond witht that radical and tha will give final product.
In first reactant cyclohexene, allylic hydrogen is abstract by N-succinamide radical, so that a new radical formed. This new radical can resonate with double bond and form stable radical. Now bromine radical can form bond this radical and form 3-bromocyclohexene. Hence 3-bromocyclohexane is formed as product.
Trending now
This is a popular solution!
Step by step
Solved in 7 steps with 7 images









