
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
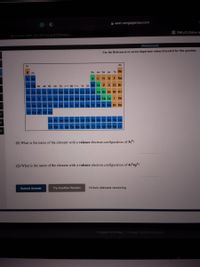
Transcribed Image Text:**Educational Content: Understanding Electron Configurations**
**Periodic Table Overview:**
- This image displays a periodic table highlighting different elements. The elements are organized into groups and periods based on their atomic structure and properties.
- The table is color-coded. Specific groups such as the noble gases in group 8A and other representative elements are easily identifiable.
**Key Questions:**
1. **What is the name of the element with a valence electron configuration of \(3s^2 3p^2\)?**
2. **What is the name of the element with a valence electron configuration of \(4s^2 4p^2\)?**
To solve these questions, refer to the periodic table and identify the elements based on their electron configurations:
- The valence electron configuration \(3s^2 3p^2\) corresponds to silicon (Si), found in period 3, group 4A of the periodic table.
- The valence electron configuration \(4s^2 4p^2\) corresponds to germanium (Ge), located in period 4, group 4A.
**Instructions:**
- Use a periodic table as a reference to understand element positioning and electron configurations.
- Attempt to answer the questions by identifying the correct elements based on given configurations.
**Interactive Elements:**
- **Submit Answer:** Button to submit your answer for evaluation.
- **Try Another Version:** Option to attempt another version of the question.
- A countdown indicating "10 item attempts remaining" to track your progress.
This educational activity helps enhance your understanding of how electron configurations relate to element identification within the periodic table.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1A H2A Li Be 8B 3A 4A 5A 6A 7A He B CN OF NE 1B 2B Al Si P S Cl Ar Na Mg 3B 4B 5B 6B 7B K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 8A Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn ** Fr Ra Ac Rf Ha Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr (1) What is the element with an electron configuration of 1s²2s²2p63s²3p64s²3d7? (2) What is the element with an electron configuration of 1s²2s²2p63s¹?arrow_forward1A 8A H 2A 3A 4A 5A 6A 7A He Li Be BCNOF Ne Na Mg 3B 4B 5B 6B 7B 88 - 1B 28 A1 Si P S CI Ar K Ca Ssc Ti Vv Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y zr Nb Mo Tc Ru Rh Pd Ag cd In Sn Sb Te I Xe Cs Ba La Hf Ta w Re Os Ir Pt Au Hg TI Pb Bi Po At Rn Fr Ra Ac Rf Ha Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa u Np Pu Am Cm Bk Cf Es Fm Md No Lr Using only the periodic table arange the following elements in order of increasing ionization energy: bismuth, antimony, arsenic, phosphorus . Lowest Highest Please answer this question according to the general rules you have learned regarding periodic trends. DO NOT base your answer on tabulated values since exceptions may occur. Drag and drop your selection from the following list to complete the answer: phosphorus antimony bismuth arsenic Prevarrow_forwardSA 4A SA 6A 7A He BCN OF Ne 18 20 Al Si P S Cl Ar Li Be Na Mg 38 48 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn Fr Ra Rc Rf Ha Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Bk Cf Es Fm Md No Lr Write the complete electron configuration for the cobalt atom. Using NOBLE GAS notation write the electron configuration for the titanium atom. 00arrow_forward
- NEED A HELP WITH THESE 06arrow_forward20arrow_forward1A Smallest H 2A 3A 4A 5A 6A 7A He BC N O F Ne Na Mg 38 48 58 6B 7B 88 18 28 Al Si P S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Li Be Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl ** Fr Ra Ac Rf Ha 8A Sn Sb Te I Xe F- Pb Bi Po At Rn Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr Arrange the following ions in order of increasing ionic radius: fluoride ion, aluminum ion, nitride ion, sodium ion. Largest Drag and drop your selection from the following list to complete the answer: A1³+ Na+ N ³-arrow_forward
- 1A 8A H 2A 3A 4A 5A 6A 7A He Li Be BCNO F Ne Na Mg 3B 4B 5B 6B 7B - 8B¬ IB 28 AI si P S CI Ar K Ca sc Ti v Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag cd In Sn Sb Te I Xe Cs Ba La Hf Ta w Re Os Ir Pt Au Hg TI Pb Bi Po At Rn ** Fr Ra Ac Rf Ha Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa u Np Pu Am Cm Bk Cr Es Fm Md No Lr Analyze the following reaction by looking at the electron configurations given below each box. Put a number and a symbol in each box to show the number and kind of the corresponding atom or ion. (Use the lowest possible coefficients. Omit states of matter.) atom atom metal ion nonmetal ion (1s²2s*) (1s 2s 2p°3s²3p®4s²3d!° 4p³ ) (1s²) (18*25 2p*3s*3p®4s*3d® 4p°)arrow_forwardled 1or this 1A 8A H 2A 7A He 3A 4A 5A 6A Li Be B CNO F Ne Na Mg 3B 4B 5B 6B 7B 8B - 1B 2B A1 Si P S CI Ar K Ca sc Ti v Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y zr Nb Mo Tc Ru Rh Pd Ag ca In Sn Sb Te I Xe Cs Ba La Hf Ta w Re os Ir Pt Au Hg TI Pb Bi Po At Rn ** Fr Ra Ac Rf Ha Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa u Np Pu Am Cm Bk Cf Es Fm Md No Lr Considering only ions with charges of +1, +2, -1 and -2, or neutral atoms, give the symbols for 4 species that are isoelectronic with the sulfide ion, S².arrow_forwardSmallest 1A Largest H2A 8A 3A 4A 5A 6A 7A He B C N O F Ne Na Mg 3B 4B 58 68 78 88 18 28 Al Si P S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Li Be Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn Fr Ra Ac Rf Ha Using only the periodic table arrange the following elements in order of increasing atomic radius: sulfur, polonium, selenium, oxygen. Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lrarrow_forward
- 1A H 2A Li Be 3A 4A 5A 6A 7A He BCN O F Ne S Cl Ar Na Mg 3B 4B 5B 6B 7B K Ca Sc Ti V 88 Cr Mn Fe Co Ni Cu Zn Ga Ag Cd In Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Fr Ra Ac Rf Ha 1B 2B Al Si P 8A Ge As Se Br Kr Sn Sb Te I Xe Pb Bi Po At Rn Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr (1) What is the complete ground state electron configuration for the chlorine atom? 1s 2s 2p 3s 3p5 (2) What is the complete ground state electron configuration for the cobalt atom?arrow_forward1A BA H 2A 3A 4A 5A 6A 7A He Li Be BCNO F Ne Na Mg 38 4B 5B 6B 7B - 8B - IB 28 AI Si PS CI Ar K Ca sc Ti v Cr Mn Fe Co Ni Cu Zn Ga Ge As se Br Kr Rb Sr Y zr Nb Mo Tc Ru Rh Pd Ag cd In Sn Sb Te I Xe Cs Ba La HT Ta w Re Os Ir Pt Au Hg TI Pb Bi Po At Rn Fr Ra Ac Rf Ha Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa u Np Pu Am Cm Bk cr Es Fm Md No Lr Using only the periodic table arrange the following elements in order of increasing atomic radius: antimony, iodine, rubidium, xenon Smallest Largestarrow_forwardWhat’s the answer to 3.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY