
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
29) when is equation is balanced with a small set of hole numbers what is the coefficient for N two? 

Transcribed Image Text:Co etticient for N ?
(5)
1でュH45)_N。 )-
A) 3
ら) 4
C) 2
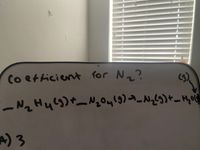
Transcribed Image Text:Co etticient for Nz?
(5)
A)3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- I need answer to this question ASAP, please. Thank you. Note: it is grade 12 chemistry so pls don't give me a university-level solution so that I can understand.arrow_forwardAt a constant temperature, which of the gases will have the highest average kinetic energy? A) H₂ B) N₂ C) O₂ D) F₂ E) All the gases will have the same average kinetic energy.arrow_forwardProblem 19.91 A standard air conditioner involves a refrigerant that is typically now a fluorinated hydrocarbon, such as CH₂F₂. An air-conditioner refrigerant has the property that it readily vaporizes at atmospheric pressure and is easily compressed to its liquid phase under increased pressure. The operation of an air conditioner can be thought of as a closed system made up of the refrigerant going through the two stages shown here (the air circulation is not shown in this diagram). Expansion chamber Liquid ▼ Liquid Part A Compression (high pressure) During expansion, the liquid refrigerant is released into an expansion chamber at low pressure, where it vaporizes. The vapor then undergoes compression at high pressure back to its liquid phase in a compression chamber. What is the sign of q for the expansion? O Positive. O Negative. Submit Expansion (low pressure) Compression chamber Part B Request Answer What is the sign of q for the compression? O Positive O Negative. Vapor Vapor 1 of…arrow_forward
- 7. Write an expression this expression as fararrow_forwardUsing the experimental data provided, determine the Activation Energy (in kJ/mol) of the following reaction: Reaction: C₂H4Br₂ (aq) + 31¯ (aq) → C₂H4 (1) + 2Br¯ (aq) + 13¯ (aq) Experimental Reaction Experiment Rate constant Temperature (L/mol s) (°C) 1 1.15x10-2 50.0 2 2.65x10-2 75.0 Constants and Conversions: Gas constants (R) are 8.314 J K-¹ mol-1 = 8.314 L kPa K-¹ mol-¹ = 0.08314 L bar K-1 mol-¹ = 0.08205 L atm K mol-1 T(K) = T(°C) + 273.15arrow_forwardWhat are some practice problems that can be solved using the Nernst equation?arrow_forward
- Why are most chemical reactions carried out either in liquid solution or in the gaseous phase? Choose all that are correct. Group of answer choices a) Reactant molecules, ions, or atoms must collide with one another in order for any chemical change to occur. b) The kinetic energy of liquids and gases is higher than for solids. c) Molecules, ions and atoms are free to orient themselves in the liquid solutions and in the gaseous phases. d) Conditions for molecular collisions are not as favorable for solids to react as they are for liquids and gasses.arrow_forward. Provide a plausible explanation for the following: Two molecules collide with sufficient energy yet do not result in the formation of products.arrow_forwardAccording to the collision theory in gaseous molecules, collision frequency is and the rate of reaction is because A) low, low, molecules are so far apart B) high, high, each collision results in a reaction C) low, low, molecules must collide before they can react D) high, relatively low, only a fraction of the collisions leads to a reaction E) low, high, molecules are moving so fast that each reaction causes many othersarrow_forward
- What is the maximum velocity in the Michaelis-Menton kinetics?arrow_forwardQuestion: What is the fundamental difference between kinetic stability and thermodynamic stability in chemical reactions, and how do they influence reaction rates?arrow_forwardIf AH is negative and (TAS ) is positive, the reaction will be energy and the AG will bearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY