
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:12:311
ull 5G
1 TikTok
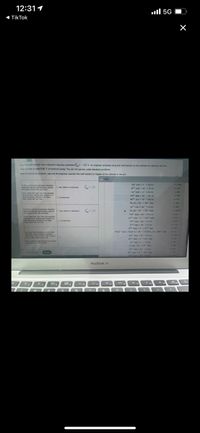
A tain haif-readtion has a standard reduction potential -1.2S V. An engineer proposes using this half-reaction at the cathode of a palvanic cel that
must provide at lest 0.80 V of electrical pewer The cell will operate under standard conditions
Note for advanced shudents assume the engineer requires this half-reaction to happen at the cathode of the cell
Ag (e) - Ag )
theremium standard reduction
hat the haee
- Dv
ys there isamu
A (a)+ A ()
Aut taal+e Au ()
1.4
, check the yes box and calulate
A (a)+ e Au (s)
1.4
no minimum
ea theck
Ba aa)+ 2e Ba (s)
2.912
Bra (0+ 2e 28 (al
Calaa) + 2 Ca (s
-3.0
herea mi
o andand
Oz (a) 2 20 (
1.
s, there isamam
he anode of ths c
Co (a) + 2e" Co ()
Ca caale Ca (aa
-4.28
4.28
check the "ye bo and caloute
emamum yr an
1.82
ne maximum
(a) + 3e"C()
C ( ()
C (a) + o 0)- 3e-CO ()+ SOr (aa)
-. 44
ng the tormationin the ALS
Det wte n
-4.11
.349
Cudt(es) Cu (
Cu" (ag)+ Cu ()
Fa lab r (
fe 2 e
re c (
elt t
2.0
4.41
nion
Check
MacBook Air
Transcribed Image Text:kamalaa.n:1 love Tt
ll 5G
1 TikTok
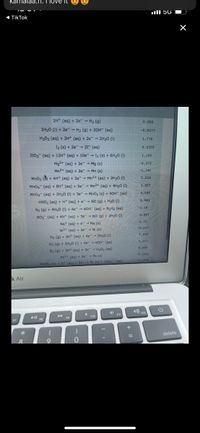
2H+ (ag) + 2e-- H2 (g)
0.000
2H20 (1) + 2e" - H2 (9) + 20H" (aq)
-0.8277
H202 (aq) + 2H+ (aq) + 2e + 2H20 (1)
1.776
I2 (s) + 2e - 21 (aq)
2103- (aq) + 12H+ (aq) + 10e- + 12 (s) + 6H20 (1)
0.5355
1.195
Mg2+ (aq) + 2e +
Mg (s)
-2.372
Mn2+ (ag) + 2e" Mn (s)
-1.185
Mno, + 4H+ (aq) + 2e - Mn2+ (aq) + 2H20 (1)
1.224
Mno4 (aq) + 8H+ (aq) + 5e" - Mn2+ (aq) + 4H20 (1)
1.507
Mno4 (aq) + 2H20 (1) + 3e - MnO2 (s) + 40H (aq)
0.595
HNO2 (aq) + H* (aq) + e- NO (g) + H20 (1)
0.983
N2 (9) + 4H20 (1) + 4e" - 40H" (aq) + N2H4 (aq)
-1.16
NO3 (aq) + 4H* (aq) + 3e" - NO (9) + 2H20 (1)
0.957
Na* (aq) + e - Na (s)
-2.71
Ni2+ (aq) + 2e- Ni (s)
-0.257
02 (9) + 4H* (aq) + 4e" - 2H20 (1)
1.229
0.401
O2 (9) + 2H2O (1) + 4e - 40H (aq)
0.695
O2 (9) + 2H* (aq) + 2e - H2O2 (aq)
-0.1262
Pb2+ (aq) + 2e"- Pb (s)
-0.3588
PbSO4 (s) + H* (aq) + 2e Pb (s) + HSOA (ag)
k Air
4)
FII
F10
F9
F7
F8
delete
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What current in ampere will be required to produce 2.369 x10-3 kg of cu from CuS04 solution in one hour? (Molar mass of Cu = 63.5g/mole) Tuno vour Answer berearrow_forward3. (a) b C. d. e. f. Given 0.01 Molar concentration Au³+ ion and 0.01 molar of Ag+ ion, and the following reaction: or Au³(aq) Ag+ (aq) -→ Au (s) -→ Ag (s) Write the balanced oxidation reduction equation for the cell E° = 1.52 V Eº = 0.80 V Which specie is being oxidized Which Specie is at the anode? Which Specie is the oxidizing agent? Which specie lost electron/s? Which specie saw an increase in oxidation state? Calculate the Ecell at non-standard condition for the reactionarrow_forwardA redox reaction used in an electrochemical cell has a negative standard free energy change, AGxn- Which statement about the redox reaction is true? O Ecel is positive; K 1 E'cel is negative; K 1 'cellarrow_forward
- Use the CTreices Tw acttss mportaIL valucs TIccucu 10I tIs qucstIon, Salt bridge A concentration cell similar to the one shown is composed of two Cd electrodes and solutions of different Cd2+ concentrations. The left compartment contains 0.395 M Cd2+ , and the right compartment contains 1.00 M Cd²+ . Calculate the cell potential for this reaction at 298 K. volts In this cadmium concentration cell, the compartment on the left is the and the compartment on the right is thearrow_forwardPlease help me how to solve this equation. Thank you very much.arrow_forwardd, e, f, & garrow_forward
- An electrochemical cell utilizes the following redox reaction NHa * (ag) + 8Ce4"(ag) + зн-0() > NO; (ag) + 8Ce3"(ag) + 10н* (ag) Where the relevant half-reactions are: NO3 - (ag) + 1OH+ (ag) + 8e- → NH4 + (ag) + 3H20 Ce**(ag) + e- > Ce3*(ag) Ecel = +0.88 V Ecel = +1.72 V 1. Calculate Ec(not under standard conditions) when [NH4 +1 = 0.35 M, [Ce**] = 0.25 M, [NO3 -1= 5.0 x10-2 M, [Ce3+] = 6.0 ×10-2 M, and the [H*] = 1.0 x10-3 M 2. How is Ecel, impacted when the concentration of H* (ag) is reduced? In other words, what happens when the pH decreases? Provide reasoning to support your answer.arrow_forwardA. From the reaction as shown below, calculate the Standard Cell Potential of a Galvanic Cell that uses the half-reactions shown below. Cr3+ + 3e- -------> Cr Eo = -0.73 V Al3+ + 3e- -------> Al Eo = -1.66 V B. A galvanic cell is made using 0.25M ZnSO4 and an unknown amount of CuSO4. The cell potential is 105V at 50oC. What is the concentration of CuSO4 in the cathode compartment? Zn(s) + Cu(aq)2+ -------> Zn(aq)2+ + Cu(s) Eo = 1.10 V Use:E = Eo - (RT/nF) + lnQarrow_forward#62arrow_forward
- Anode Cathoode Zn (NOg)a Ag NOR Fet the lealanced RHR ,OHR , and overall reaution of lhe vooltaic cellarrow_forwardPart A What mass of Cu(s) is electroplated by running 30.0 A of current through a Cu* (aq) solution for 4.00 h? Express your answer to three significant figures and include the appropriate units. • View Available Hint(s) 142 g Submit Prevloue Anewere v Correct Part B How many minutes will it take to electroplate 55.1 g of gold by running 5.00 A of current through a solution of Au* (aq)? Express your answer to three significant figures and include the appropriate units. • View Available Hint(s) Tempjetes Symbole undo rego teset keyboard shortouts Heip 36.3 min Submit Prevloue Anewers X Incorrect; Try Again; 2 attempts remainingarrow_forwardConsider a fuel cell that uses methane ("natural gas") as fuel. The reaction is CH4 + 2O2 → 2H2O + CO2 (a) The steps of this reaction areat electrode: CH4 +2H20 -C02 +8H+ +8e-; at + electrode: 202 +8H+ +8e--4H20.What is the voltage of the cell?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY