
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:TO McGraw Hill Campus
Cn
|||
XA
A ALEKS- Elizabeth Galaz - Learn
x Calculate the Molar Mass of Ace! x +
https://www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-liG1Sp4C1qHE9vg-2-exSXq493r_tJ2gZs0a-_xkRSR8a7T-... A
O CHEMICAL REACTIONS
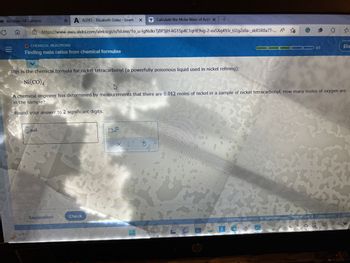
Finding mole ratios from chemical formulae
This is the chemical formula for nickel tetracarbonyl (a powerfully poisonous liquid used in nickel refining):
Ni(CO)
mol
Explanation
A chemical engineer has determined by measurements that there are 0.012 moles of nickel in a sample of nickel tetracarbonyl. How many moles of oxygen are
in the sample?
Round your answer to 2 significant digits.
Check
X
Search
3/5
LOO
hp
© 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center
V
Eliz
TUNIN
Acces
D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- college.com/course.html?courseld=15539865&Heplb=67br| Sugar O Shop | Origami Owl Wholesale Glass... 5 The Corinthian Ho... Personalized Invi... TUII Z TJ TUT CTLIVT Course Homearrow_forwardS Schoology D persuasive essay-outline x O Untitled presentation - G 9 Unit 8 Moles and Molar G if you are given 743 gr. in A aldine.schoology.com/common-assessment-delivery/start/4607591875?action=onresume&submissionld=373201347 prg Bookmarks O Papa's Freezeria -P. G graduation cap - G. O Conair Scunci Cont. O Untitled presentatio. Unit 8 REVIEW of solving steps If you are given 9.47 x 1024 formula units of LiF, how many moles do you have of this imaginary compound? O 0.0636 O 1.57 O 15.7 O 6.02 x 1023 11 12 13 14 15 16 17 18 19 20 Next US Garrow_forwardcalula te bollowing 1a) ob (2) Num ber 6molecules in 0-250 moles NH3arrow_forward
- be Maps Netflix in other Co... Nhttps://lms.naz.ed... Rochester to Seat... rus What is the mass of one formula unit of calcium cyanide, Ca(CN)2? amu Question #6 t How would you calculate (see below) the number of formula units of Ca(CN)2 of in 7 moles of Ca(CN)2? C V port 7 x formula mass (A) 7 x molar mass (B) 7 x Avogardro's Number (C) 7 7 formula mass (D) molar mass (E) Submit Answers 7 Avogardro's Number (F) McKee Next Previous 60arrow_forwardcan u help me w this problemarrow_forwardRemaining Time: 1 hour, 01 minute, 47 seconds. * Question Completion Status: QUESTION 2 The number of water molecules in 2.3 mg of water is O 6.02 x 1023 O 77.0 x 1023 O 7.7 x 1023 O 7.7 x 1019 none of the above QUESTION 3 A ctudent makes a colution bu discolvina 254a ofNDOH int0 450 gof water Wh. Click Save and Submit to save and submit. Click Save All Answers to save all answer ere to search DELLarrow_forward
- Question 16 of 96 Submit Determine the number of atoms of O in 54.1 moles of Cr3(PO4)2. | atoms 1 2 4 6. C 7 +/- x 10 0 Tap here or pull up for additional resources LO 00arrow_forwardDo b and c onlyarrow_forwardmmunity College X x || X D2L ALEKS Learning Module 8 (due A X A ALEKS- Jessica Sceusa - Learn X G Write the empirical formula for a x + www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-lvWyv8WYLP6W0cqJcWJdIACROQwyw24GWHInCX70-Q0WaoxQiZ3DF-1EgBJkRS45QbFh_mdu6pQMT19A08TrT_6p5Ces O ||| O STATES OF MATTER Using the combined law gas Student - Home kPa For many purposes we can treat ammonia (NH3) as an ideal gas at temperatures above its boiling point of -33. °C. Suppose the temperature of a sample of ammonia gas is lowered from 19.0 °C to -25.0 °C, and at the same time the pressure is changed. If the initial pressure was 0.30 kPa and the volume decreased by 40.0%, what is the final pressure? Round your answer to 2 significant digits. Explanation Check X10 X K 0/5 © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Centarrow_forward
- ber.ca - Search G SUSC File Paste 35:51 Xcut Home View Copy X Untitled-Paint 4127, 295 https://ca.bbcollab.com/collab/ui/session/playback Select Announcements Crop Resize Rotate A f P Brushes nga = MGA Colors Clipboard Tooli Shapes What mass of gallium oxide, Ga₂O3, can be prepared from 29.0g of gallium metal? Use the equation: W X WOD044 Outline DOOOO ODRE 3+000- MMG 4Ga + 30₂2Ga₂03 Blackboard Collaborate 12 4506 2514px 35:52 X > = 29g - 0. 69.729 / molarrow_forwardThe percentage composition by mass (to the nearest 0.1) of C in sugar, C8H10O7, is ______________.arrow_forwardhttps://cfisd.schoology.com/common-assessment-delivery/start/4981371910?action=Donresum kick Bailar Conjugation |. Fraction Calculator O A Land of Permane... Basic Stoichiometry and Limiting Reactant Quiz -L Assuming 11.5 L of CO2 are produced from this reaction, how many moles of CaCO3 are needed? Be Caco3 - Cao + CO2 I Type here to search !!!arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY