
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Hi I need help finding the actual moles used please:)
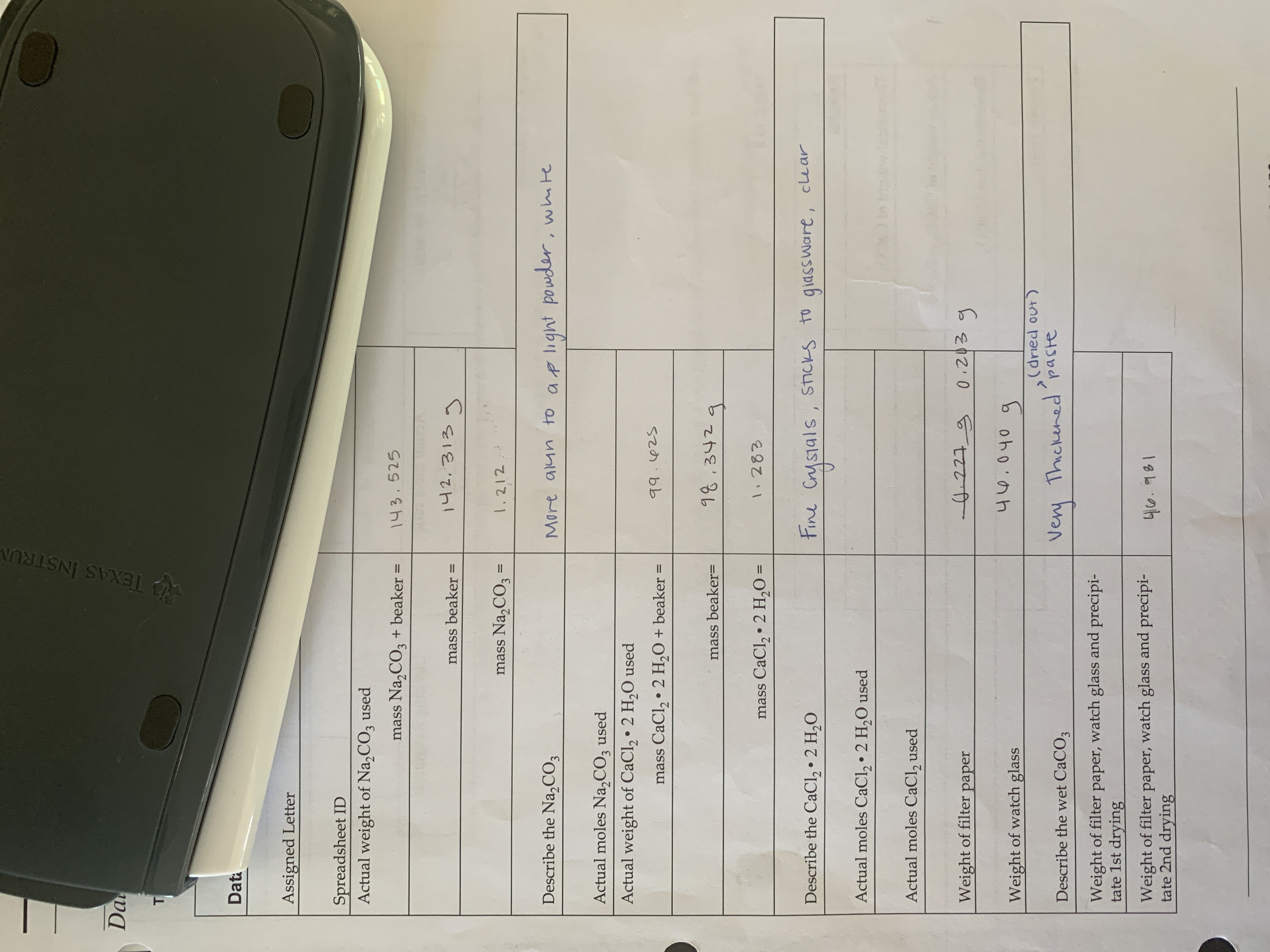
Transcribed Image Text:TEXAS INSTRUM
Da
Dat
Assigned Letter
Spreadsheet ID
Actual weight of Na,CO, used
mass Na,CO3 + beaker
143.525
142.313g
mass beaker
mass Na,CO3 =
1,212
%3D
Describe the Na,CO3
More alun to ap light powder, whte
Actual moles Na,CO3 used
Actual weight of CaCl, • 2 H,0 used
mass CaCl, • 2 H,O + beaker =
%3D
mass beaker=
mass CaCl, • 2 H,0 =
%3D
Describe the CaCl, • 2 H,0
Fine Crysials, StIcks to
giassware, cle ar
Actual moles CaCl, • 2 H,O used
Actual moles CaCl, used
Weight of filter
paper
Weight of watch glass
(dried out)
Very Thickened paste
eery
Describe the wet CaCO3
Weight of filter paper, watch glass and precipi-
tate 1st drying
Weight of filter paper, watch glass and precipi-
tate 2nd drying
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Apps e Ch 9: Homework 0/1 E Question 12 of 12 Methyl alcohol (CH3OH) is made by the reaction of carbon monoxide and hydrogen in the presence of certain metal oxide catalysts. CO(g) + 2H2(g) –→CH3OH(I) X Incorrect. Did you use the proper mole ratios? How much alcohol can be obtained by reaction of 31.2 g of CO and 9.57 g of H2? 375 Hint X Incorrect. Did you use the proper mole ratio? | 理 Type here to searcharrow_forwardStuck on how to solve for theoretical moles. (#1 a)arrow_forwardPls help, sorry for the troublearrow_forward
- How many moles are in 9.54 x10^22 formula units of Li3P *with correct number of sig figsarrow_forwardFOR 2/8 X . OL Unit 4 Review Choice Board O 4.3.SC PL Mole Calculations goformative.com/formatives/6000773af6eb3353bf25db40 Gabbi Fraychine A healthly person breathes out approximately 0.020 L of carbon dioxide in one breath. How many moles of carbon dioxide is in one breath? The molar mass of carbon dioxide is 44.01 g/mol. Show Your Work SATISFACTORYarrow_forwardPart D Calculate the moles of C2 H6 in 3.76x1023 molecules of C2 H6. Express your answer using three significant figures. moles of C2 H6 Submit Previous Answers Request Answer X Incorrect; Try Again; 4 attempts remaining Provide Feedback < Previousarrow_forward
- An unknown organic compound containing 3 COOH groups has a molar mass of 110.01 g/moles. What is the theoretical NE value? Answer should be a whole number.arrow_forwardHow many grams of H2SO4 are present in 1.9*1026 formula units of H2SO4 ? Round your answer to 1 decimal place. Your Answer: Answer unitsarrow_forwardPlease advisearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY