
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Help
Transcribed Image Text:A Moving to another question will save this response.
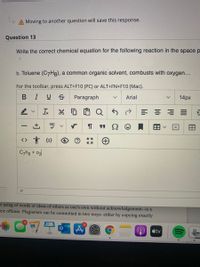
Question 13
Write the correct chemical equation for the following reaction in the space p
b. Toluene (C7H8), a common organic solvent, combusts with oxygen...
For the toolbar, press ALT+F10 (PC) or ALT+FN+F10 (Mac).
BIUS
Paragraph
Arial
14px
In
ABC
<> Ť {i}
C7ha + o2
e using of words or ideas of others as one's own without acknowledgement--is a
mic offense. Plagiarism can be committed in two ways: either by copying exactly
tv
田
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- If the caffeine concentration in a particular brand of soda is 3.37 mg/oz, drinking how many cans of soda would be lethal? Assume that 10.0 g of caffeine is a lethal dose, and there are 12 oz in a can. cans of soda:arrow_forwardcan you help with 16, 17, and 18 as well please?arrow_forwardInjecting an arterial fluid which is hypertonic to the moisture level of the body will ___. add more water to the tissues remove water from the body's tissues bring salt and sugar molecules into the capillaries, they are removed in the venous drainage cause decomposition to progress fasterarrow_forward
- 13. 15 g of sodium chloride is diluted in 1 L of sterile water for injection (SWFI). What is the final percent strength (w/v)? 14. How much stock solution of hydrogen peroxide 10% solution will you need to make 300 mL of hydrogen peroxide 3% solution? 15. 500 mg of Vibramycin IV® is diluted in 500 mL LR to be administered over 4 hours. How much drug will be administered per hour?arrow_forward5. Explain the effect of the IMFA on the properties of liquids and list at least two (2) examples of each. Effect of the IMFA on the Properties of Properties of Liquids Examples Liquids 1. 1. 1. 2. 2. 2. 1. 2. 3. 3. 1. 2. 5. 1. 2. 6. 1. 2. 7. 7. 1. 2. 2. 1. 4. 6. 4. 5.arrow_forwardNeed help with #2. (Info in #1 is needed to solve for #2)arrow_forward
- What is the [H30*] concentration for a solution where [OH] = 2.3 x 103 M? Kw = 1.0 x 1014 = [H3O*][OH] %3D A. 4.3 x 10 12 M B. 1.2 x 10 13 M C. 2.8 x 10 13 M D. 7.9 x 10 12 M E. 4.6 x 103 Marrow_forwardC. SHarrow_forwardMrs A is currently taking Mucogel suspension at a daily dose of 10 mL after her three meals andat bedtime. How much magnesium hydroxide will Mrs A have taken after 5 days of compliant useof Mucogel? (Mucogel contains magnesium hydroxide 195 mg and dried aluminium hydroxide220 mg/5 mL.)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY