
Principles of Modern Chemistry
8th Edition
ISBN: 9781305079113
Author: David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
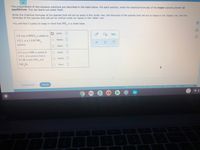
Transcribed Image Text:The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at
equilibrium. You can leave out water itself.
Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the
formulas of the species that will act as neither acids nor bases in the 'other' row.
You will find it useful to keep in mind that NH, is a weak base.
acids:
1.0 mol of HNO, is added to
1.0 L of a 1.0 M NH,
bases: O
solution
other:
0.2 mol of HBr is added to
O acids:
1.0 L of a solution that is
O. bases:
0.7 M in both NH, and
NH Br.
other:
Check
Explanation
2021 McGraw-Hi Education. All Rights Reserved. Terms of Use Privacy Accessibility
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Write a formula for the conjugate base formed when each of the following behaves as a Brnsted acid: a. HSO3 b. HPO42 c. HClO3 d. CH3NH3+ e. H2C2O4arrow_forwardWhich of the following substances are acids in terms of the Arrhenius concept? Which are bases? Show the acid or base character by using chemical equations. a P4O10 b Na2O c N2H4 d H2Tearrow_forwardA base is a substance that dissociates in water into one or more ______ ions and one or more ________. a.hydrogen . . . anions b.hydrogen . . . cations c.hydroxide . . . anions d.hydroxide . . . cationsarrow_forward
- Write equations to illustrate the acid-base reaction when each of the following pairs of Brnsted acids and bases are combined: Acid Base a.HOCl H2O b.HClO4 NH3 c.H2O NH2 d.H2O OCl e.HC2O4 H2Oarrow_forwardWhen perchloric acid ionizes, it makes the perchlorate ion, ClO4. Draw the Lewis electron dot symbol for the perchlorate ion.arrow_forwardYou are asked to determine whether an unknown white solid is acidic or basic. You also need to say whether the acid or base is weak or strong. You are given the molar mass of the solid and told that it is soluble in water. Describe an experiment that you can perform to obtain the desired characteristics of the white solid.arrow_forward
- Aluminum chloride, AlCl3, reacts with trimethyl-amine, N(CH3)3. What would you guess to be the product of this reaction? Explain why you think so. Describe the reaction in terms of one of the acid base concepts. Write an appropriate equation to go with this description. Which substance is the acid according to this acidbase concept? Explain.arrow_forwardHow would you compare the strengths of two weak acids experimentally? By looking up information in a table or a handbook?arrow_forwardClassify each of the following statements as true or false: aAll Brnsted-Lowry acids are Arrhenius acids. bAll Arrhenius bases are Brnsted-Lowry bases, but not all Brnsted-Lowry bases are Arrhenius bases. c HCO3 is capable of being amphoteric. d HS is the conjugate base of S2. eIf the species on the right side of an ionization equilibrium are present in greater abundance than those on the left, the equilibrium is favored in the forward direction. f NH4+ cannot act as a Lewis base. gWeak bases have a weak attraction for protons. hThe stronger acid and the stronger base are always on the same side of a proton transfer reaction equation. iA proton transfer reaction is always favored in the direction that yields the stronger acid. jA solution with pH=9 is more acidic than one with pH=4. kA solution with pH=3 is twice as acidic as one with pH=6. lA pOH of 4.65 expresses the hydroxide ion concentration of a solution in three significant figures.arrow_forward
- Arsenic acid (H3AsO4) is a moderately weak triprotic acid. Write equations showing its stepwise dissociation. Which of the three anions formed in these reactions will be the strongest Brnsted base? Which will be the weakest Brnsted base? Explain your answers.arrow_forwardAccording to the Arrhenius theory of acids and bases, how do you recognize an acid and a base?arrow_forwardCalculate the concentration of all solute species in each of the following solutions of acids or bases. Assume that the ionization of water can be neglected, and show that the change in the initial concentrations can be neglected, Ionization constants can be found in Appendix H and Appendix I. (a) 0.0092 M HCIO, a weak acid. (b) 0.0784 M C6H5NH2, a weak base. (c) 0.0810 M HCN, a weak acid. (d) 0.11 M (CH3)3N, a weak base. (e) 0.120 M Fe(H2O)62+ a weak acid, Ka=1.6107arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning