
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
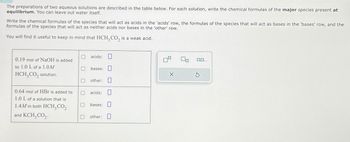
Transcribed Image Text:The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at
equilibrium. You can leave out water itself.
Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the
formulas of the species that will act as neither acids nor bases in the 'other' row.
You will find it useful to keep in mind that HCH3CO2 is a weak acid.
0.19 mol of NaOH is added
to 1.0 L of a 1.0M
HCH, CO₂ solution.
☐ acids:
bases:
☐ other: ☐
0.64 mol of HBr is added to
O
acids:
1.0 L of a solution that is
1.4M in both HCH,CO₂
bases:
and KCH, CO2.
other:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 11 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following represents the hydration of acetic acid (a weak electrolyte)? O CH3COOH () H20 CH3+ (aq) + COOH (aq) O CH3COOH () H20 CH3COO (aq) + H* (aq) H20 O CH3COOH () CH3COO (aq) + H* (aq) H20 CH3COOH () CH3COOH (aq)arrow_forwardEach row of the table below describes an aqueous solution at 25 °C. The second column of the table shows the initial components of the solution. • Use the checkboxes in the third column to explain the type of the initial solution. The fourth column describes a change in the solution. • Use the fifth column to predict how the change in the solution will change its pH. initial type solution initial components (check all that apply) acidic basic neutral acidic basic neutral acidic basic neutral acidic basic neutral A B 09 C D H₂O H₂O H₂O, HCIO H₂O, HCIO4 0000000 change add HI add KI add NaClO add NaOH effect of change on pH (check one) O pH higher O pH lower OpH the same O pH higher O pH lower OpH the same O pH higher O pH lower O pH the same O pH higher O pH lower O pH the samearrow_forwardWhat mass of sodium acetate must be added to 435 mL of water to give a solution with pOH = 4.65? Kb of acetate is 5.68 x 10-10.arrow_forward
- If 8.00 mL of a different hydrochloric acid solution had a known concentration of 0.3456 M, how many mL of the 0.1232M NaOH solution would be required to reach the equivalence point? (3 sig figs)arrow_forwardA substance is mixed with water and it donates 0.4% of its H+ ions. Which of the following BEST describes the substance?arrow_forwardRow 1: Your answer is incorrect. • Row 2: Your answer is incorrect. Row 4: Your answer is incorrect. The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that NH, is a weak base. 1.3 mol of HI is added to 1.0 L of a 1.3MNH, solution. 0.22 mol of HNO, is added to 1.0 1. of a solution that is 1.1M in both NH, and NH Br. ✔ acids: NH, H,O bases: 11,0, NHL, other: I acids: NH H bases: NH, other: NO, Br a S da X 0.0.arrow_forward
- when solutions of Na2S and FeCI3 are mixed, What is the for the solid formed?arrow_forwardJosephine reacts 10.0 mL of 0.50 M HCI with 10.0 mL of 0.50 M KOH. The solution experienced a temperature rise of six degrees Celcius. If she doubles the volume of acid and base she used (while not changing the concentrations), would the temperature rise be half of the original AT (3 C), the same as the original AT (6 C) or twice that of the original AT (12 C) ? Justify your answer. 5. List two things that are necessary for an effective collision at the molecular level. 6. List one way a student can increase the rate that his rock salt (magnesium sulfate pieces) reacts with acid.arrow_forwardIn the following acid-base equilibria of weak acids in water, label the acid (A), the base (B), the conjugate acid (CA), and the conjugate base (CB). HCIO,(aq) + H,O(1) = H,O*(aq) + C1O, (aq) H₂CO3(aq) + H₂O(1) H₂O+ (aq) + HCO3(aq) H,O(1) +CH,NH (aq) = CH,NH,(aq) + H,O*(aq) O CH₂COOH(aq) + H₂O(1) ⇒ CH₂COO¯(aq) + H₂O+ (aq) O B Answer Bank CA A CBarrow_forward
- student recorded the temperature change and calculated the enthalpy of neutralization. chemists. Explain why and how (higher or lower) the temperature will be different. Identify the who a change that will be from that by the other two 1.10 M HCI (equal to his NaOH added 50.0 mL of M HCl to her NaOH Each coffee cup (Part B). Brett added 50,0 mL of HCI to his of 45.5 mL of 3. Part B. 50.0 mL of M in separate Styrofoam Experiment 25 2arrow_forwardEach row of the table below describes an aqueous solution at 25 °C. The second column of the table shows the initial components of the solution. • Use the checkboxes in the third column to explain the type of the initial solution. The fourth column describes a change in the solution. • Use the fifth column to predict how the change in the solution will change its pH. alo initial type solution initial components (check all that apply) effect of change on pH change (check one) O acidic pH higher H,0 basic add H C10, O pH lower A O neutral O pH the same acidic O pH higher H,0 add Na ClO, B O basic O pH lower O neutral pH the same O acidic pH higher Н, 0, КОН V basic add H Br O pH lower O neutral O pH the same O acidic O pH higher D Н,о, КОН O basic add K Br O pH lower O neutral pH the same Explanation Check © 2021 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accessibilityarrow_forwardA 0.251 g sample of impure NaOH requires 17.6 mL of 0.2903 M HCl for neutralization. What is the percent of NaOH in the sample, by weight?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY