
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
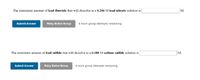
Transcribed Image Text:The maximum amount of lead fluoride that will dissolve in a 0.206 M lead nitrate solution is
М.
Submit Answer
Retry Entire Group
6 more group attempts remaining
The maximum amount of lead sulfide that will dissolve in a 0.180 M sodium sulfide solution is
М.
Submit Answer
Retry Entire Group
6 more group attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Decide whether a chemical reaction happens in either of the following situations. If a reaction does happen, write the chemical equation for it. Be sure your chemical equation is balanced and has physical state symbols. situation A strip of solid magnesium metal is put into a beaker of 0.027M PdCl₂ solution. Explanation chemical reaction? A strip of solid palladium metal is put into a beaker O yes of 0.046M MgBr₂ solution. O no O yes O no ** Check 80 000 chemical equation 0 MacBook Air .. ローロ X 00 0 Ś © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibilit DII FO TODarrow_forwardChapter 5 Algorithmic Question 53 34 of 54 Review I Constants I Periodic Table Part A Balance the chemical equation given below, and determine the number of milliliters of 0.00300 M phosphoric acid required to neutralize 35.00 mL of 0.00150 M calcium hydroxide. Ca(OH)2(aq) + H3PO4(aq) → Ca3(PO4)2(s) + H20() O 2.36 mL O 17.5 mL 26.2 mL O 11.7 mL Submit Request Answer Next > Provide Feedback MacBook Proarrow_forwardReferences Use the References to access important values if needed for this question. In the laboratory you dissolve 17.8 g of sodium hydroxide in a volumetric flask and add water to a total volume of 375 mL. What is the molarity of the solution? М. What is the concentration of the sodium cation? М. What is the concentration of the hydroxide anion? М. Submit Answerarrow_forward
- In the reaction between lead (II) iodide and potassium nitride to yield lead (II) nitride and potassium iodide, the coefficient in front of the potassium iodide is __________. 3 5 6 2 4arrow_forward7. Which reaction will produce a precipitate in aqueous solution? CaCl2 + Na2CO3 → c) H2SO4 + KOH → a) d) 2NH.CI + K2SO4 → b) e) HNO3 + NAOH → NaOH + H2S →arrow_forwardThe maximum amount of chromium(III) phosphate that will dissolve in a 0.145 M potassium phosphate solution is M. Submit Answer Retry Entire Group 2 more group attempts remainingarrow_forward
- Write the balanced net ionic equation for the precipitation of nickel(II) carbonate from aqueous solution: Include states of matter in your answer. Submit Answer Retry Entire Group + 9 more group attempts remaining -> Previousarrow_forwardYou need to make an aqueous solution of 0.169 M magnesium iodide for an experiment in lab, using a 500 mL volumetric flask. How much solid magnesium iodide should you add? grams Submit Answer Retry Entire Group 9 more group attempts remainingarrow_forwardions submit when you are finished with th uestions carefully, some questions have molar mas Question 31 Which of the following is evidence that a chemical reaction has occurred? O A solid is formed in the reaction. O All of these are evidence that a chemical reaction has occured. zzes O Gas bubbles are observed in the reaction. Modules Chat The temperature of the reaction goes up by 20 degrees. Google Drive O A color change is noticed in the reaction vessel. * Previous MacBo esc F1 吕0 F3 F4 F5 24 T. # 3 ※江arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY