
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:ions
submit when you are finished with th
uestions carefully, some questions have molar mas
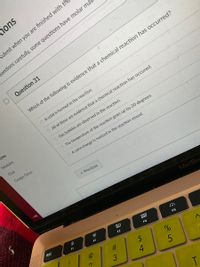
Question 31
Which of the following is evidence that a chemical reaction has occurred?
O A solid is formed in the reaction.
O All of these are evidence that a chemical reaction has occured.
zzes
O Gas bubbles are observed in the reaction.
Modules
Chat
The temperature of the reaction goes up by 20 degrees.
Google Drive
O A color change is noticed in the reaction vessel.
* Previous
MacBo
esc
F1
吕0
F3
F4
F5
24
T.
# 3
※江
Expert Solution
arrow_forward
Step 1
Chemical reaction involves reactant and products in which reactant react to form products .
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The boxes shown below represent a set of initial conditions for the reaction: Draw a quantitative molecular picture that shows what this system looks like after the reactants are mixed in one of the boxes and the system reaches equilibrium. Support your answer with calculations. Consider an equilibrium mixture of four chemicals (A, B, C, and D, all gases) reacting in a closed flask according to the foll owing equation: A+BC+D a. You add more A to the flask. How does the concentration of each chemical compare to its original concentration after equilibrium is re-established? Justify your answer. b. You have the original set-up at equilibrium, and add more D to the flask. How does the concentration of each chemical compare to its original concentration after equilibrium is re-established? Justify your answer.arrow_forward. Before two molecules can react, chemists envision that the molecules must first collide with one another. Is collision among molecules the only consideration for the molecules to react with one another?arrow_forward. Explain what it means that a reaction has reached a state of chemical equilibrium. Explain why equilibrium is a dynamic state: Does a reaction really “stop” when the system reaches a state of equilibrium? Explain why, once a chemical system has reached equilibrium, the concentrations of all reactants remain constant with time. Why does this constancy of concentration not contradict our picture of equilibrium as being dynamic? What happens to the rates of the forward and reverse reactions as a system proceeds to equilibrium from a starting point where only reactants are present?arrow_forward
- . For the reaction N2(g)+3H2(g)2NH3(g), list the types of bonds that must be broken and the type of bonds that must form for the chemical reaction to take place.arrow_forwardThe characteristics of four reactions, each of which involves only two reactants, are given. For each of the following pairs of the preceding reactions, compare the reaction rates when the two reactants are first mixed by indicating which reaction is faster. a. 1 and 2 b. 1 and 3 c. 1 and 4 d. 2 and 3arrow_forwardFor the simple reaction 2H2(g)+O2(g)2H2O(l)list the types of bonds that must be broken and the types of bonds that must form for the chemical reaction to take place.arrow_forward
- Classify each of the reactions according to one of the four reaction types summarized in Table 18.1. (a) Fe2O3(s) + 2 Al(s) 2 Fe(s) + Al2O3(s) rH = 851.5 kj/mol-rxn rS = 375.2 J/K mol-rxn (b) N2(g) + 2 O2(g) 2 NO2(g) rH = 66.2 kJ/mol-rxn rS = 121.6 J/K mol-rxn TABLE 18.1 Predicting Whether a Reaction Will Be Spontaneous Under Standard Conditionsarrow_forwardDetermine rxnH 25 C for the following reaction: NO g O2 g NO2 g This reaction is a major participant in the formation of smog.arrow_forwardHow do chemists envision reactions taking place in terms of the collision model for reactions? Give an example of a simple reaction and how you might envision the reaction’s taking place by means of a collision between the molecules.arrow_forward
- Classify each of the reactions according to one of the four reaction types summarized in Table 18.1. (a) C6H12O6(s) + 6 O2(g) 6 CO2(g) + 6 H2O() rH = 673 kj/mol-rxn rS = 60.4 j/K mol-rxn (b) MgO(s) + C(graphite) Mg(s) + CO(g) rH = 490.7 kJ/mol-rxn rS = 197.9 J/K mol-rxn TABLE 18.1 Predicting Whether a Reaction Will Be Spontaneous Under Standard Conditionsarrow_forwardSubstances that poison a catalyst pose a major concern for many engineering designs, including those for catalytic converters. One design option is to add materials that react with potential poisons before they reach the catalyst. Among the commonly encountered catalyst poisons are silicon and phosphorus, which typically form phosphate or silicate ions in the oxidizing environment of an engine. Group 2 elements are added to the catalyst to react with these contaminants before they reach the working portion of the catalytic converter. If estimates show that a catalytic converter will be exposed to 625 g of silicon during its lifetime, what mass of beryllium would need to be included in the design?arrow_forwardFor a precipitation reaction to be useful in a gravimetric analysis, the product of the reaction must be insoluble. Is Kc1,1 or 1 for a useful precipitation reaction?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemical Principles in the LaboratoryChemistryISBN:9781305264434Author:Emil Slowinski, Wayne C. Wolsey, Robert RossiPublisher:Brooks Cole
Chemical Principles in the LaboratoryChemistryISBN:9781305264434Author:Emil Slowinski, Wayne C. Wolsey, Robert RossiPublisher:Brooks Cole General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Brooks Cole

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning