
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
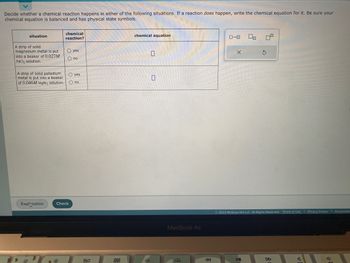
Transcribed Image Text:Decide whether a chemical reaction happens in either of the following situations. If a reaction does happen, write the chemical equation for it. Be sure your
chemical equation is balanced and has physical state symbols.
situation
A strip of solid
magnesium metal is put
into a beaker of 0.027M
PdCl₂ solution.
Explanation
chemical
reaction?
A strip of solid palladium
metal is put into a beaker
O yes
of 0.046M MgBr₂ solution. O no
O yes
O no
**
Check
80
000
chemical equation
0
MacBook Air
..
ローロ
X
00 0
Ś
© 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibilit
DII
FO
TOD
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- novel process for obtaining magnesium from sea water involves several reactions. Write a balanced chemical equation for each step of the process. (Use the lowest possible whole number coefficients. Include states-of-matter under the given conditions in your answer.) In the third step, calcium hydroxide (mixed with seawater) reacts with dissolved magnesium chloride to yield solid magnesium hydroxide and aqueous calcium chloride. In the fourth step, solid magnesium hydroxide is added to an aqueous hydrochloric acid solution, producing dissolved magnesium chloride and liquid water. magnesium chloride is melted and this liquid is electrolyzed to yield liquid magnesium metal and diatomic chlorine gas.arrow_forwardO Kf will change depending on what solute is dissolved in the solvent. QUESTION 11 Which term can be used to describe the process in the reaction below? 2 NaHCO3 (s) Na2CO3 (s) + H2O (g) + CO2 (g) Dissociation Precipitation Hydration Decomposition QUESTION 12 Which of the following would NOT be considered valid sources of error in a laboratory experiment? Click Save and Submit to save and submit. Cick Suve All Answers to save all ansicersarrow_forwardParaffin wax vapor reacts with oxygen gas to form carbon dioxide gas and water vapor. Paraffin wax is a mixture of hydrocarbons having a general chemical formula of CnH2n+2 where n is at least 16. Assume that for this wax n=30 1. Write the symbolic form of this chemical reaction. 2. Classify this chemical reaction and write the general form of this type of reaction. 3. If the wax is a solid at room temperature, why does the reaction state that it is a gas? 4. Explain why the reaction stopped after the glass was placed over the candle. 5. Explain what was on the inside surface of the glass and where it came from.arrow_forward
- The reactants below undergo a single-replacement reaction. Choose the balanced reaction equation below that correctly predicts the products. K + MgBr2 → 2 K + MgBr2 → 2 KBr + Mg K + MgBr2 → KMgBr2 K + MgBr2 → Br2 + KMg K + MgBr2 → KBr2 + Mgarrow_forwardWrite the balanced formula equation for this reaction: HCl + NH3 → Enter your answer as the sum of coefficients. Determine the balanced total ionic equation for the same reaction and enter the sum of the coefficients.arrow_forwardSilver Nitrate Solution, AgNO3 Anion Reaction With AgNO3 (ionic equations for observed reactions) Solubility in NH3 Solubility in nitric acid (HNO3) Cl- Br- I- SO32- CO32-arrow_forward
- Which of the following reactions is an example of a single replacement reaction? HCl + NaOH → NaCl + H2O N2 + 3 H2 → 2 NH3 Mg + 2HNO3 → Mg(NO3)2 + H2 CaCO3→ CaO + CO2arrow_forwardThank youarrow_forwardIn the reaction of magnesium metal with hydrochloric acid, how do you determine when the magnesium metal has reacted completely? Select all that apply. Mg (s) + 2 H* (aq) → Mg²+ (aq) + H2 (g) Select one or more: All of the water has evaporated. The dilute Hcl is gone. The magnesium metal is gone. Gas bubbles are no longer produced.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY