
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:000
lendar
Inbox
History
0886
Studio
Help
个
Study.com
Upswing
EAB Navigate
Esc
Microsoft Teams
classes
Microsoft Teams
meetings
BryteWave Course
Materials
Type here to search
F1
F2
@
Select all that apply:
F3
#
bent
trigonal pyramidal
trigonal planar
tetrahedral
O
M
F4
100
$
F5
C
%
5
19
F6
>>
A
CO
C
F7
&
F8
7
7
F9
83°F Mostly
Transcribed Image Text:ons
com
ing
10
Navigate
osoft Teams
ses
crosoft Teams
etings
yteWave Course
aterials
e here to search
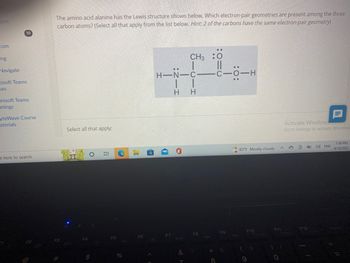
The amino acid alanine has the Lewis structure shown below. Which electron-pair geometries are present among the three
carbon atoms? (Select all that apply from the list below. Hint: 2 of the carbons have the same electron-pair geometry)
F3
Select all that apply:
F4
O
$
0
Ei
F5
%
3'
F6
A
H-N-C
| |
H H
(7
F7
..
CH3 :O
-
F8
& 7
7
||
C-
-C
F9
*8
8
:O:
-0-H
83°F Mostly cloudy
(
F10
9
)
F11
0
O
Activate Windows
Go to Settings to activate Windows
F12
F
ENG
Ih
1:56 PM
9/18/2022
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Plz do Asap....! Que 9arrow_forward1. How many electrons does carbon have in its valence shell? 2. Carbon most frequently forms bonds with 3. Draw methane (CH4) using electron distribution diagrams. Example: Neon (₁oNe) 4. 5. Label the isomers: H HC H HH HH CH OH Hydroxyl H H H H H HH H C-H H are organic molecules that only consist of carbon and hydrogen. H c-c 6. Draw a line to connect the functional group to its structure: H SH с-с H - OH WA A -N H H Carbonyl Carboxyl Amino Sulfhydry! 11 Phosphate -P-0 0- Methyl andarrow_forward5 organic chemistry , please draw.arrow_forward
- How many o bonds are in the structure of the organic molecule below? H ***** :O: -N: C Tap here or pull up for additional resources :0: IC :Z—H N Н C 010 с N: Z: H N Question 8 of 18 C-Harrow_forward6) Which of the following molecules is hydrophobic / nonpolar A. B.) C. "H (_ H₂N- H A) H-J с /\ I. # H T H U=O NH₂ с Î · OH н H D. •C- N' H. H₂C ç HH H CH3 н E) None of these molecules are hydrophobic H C-OH H ال ہے H C/OHarrow_forwardit is asking for the condensed form but we did not practice condensed form in class so i’m confused how it would look like for this compoundarrow_forward
- Is there anything wrong with this Lewis Structure of ICI4" ? If no, explain why not. If yes, explain why it is wrong and how to fix it. Edit View Insert Format Tools Table 12pt v Paragraph BIU esc * 23 % @ 2 3 4arrow_forwardDraw the lewis structure of SO2 ( best resonance) and 2nd best resonance showing the shape and bond angles of each one, the 3D structure with polar bonds or bonds dipole of each one and the molecular polarity. Here is an example of what I want the answer to be like:arrow_forwardF3 Use the following Lewis diagram for propene to answer the questions: HI 11 2 3 Remember that geometry refers to the geometry defined by the atoms, not the electron pairs. The geometry about atom 1 is The geometry about atom 2 is The geometry about atom 3 is Submit Answer $ 4 R F F4 % H 5 Use the References to access important values if needed for this question. Retry Entire Group F5 T G Cengage Learning Cengage Technical Support ^ 6 MacBook Air F6 9 more group attempts remaining Y H & 7 U * 00 8 FB ( 9 K F9 0 P Previous Email Instructor F11arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY