
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
7,8
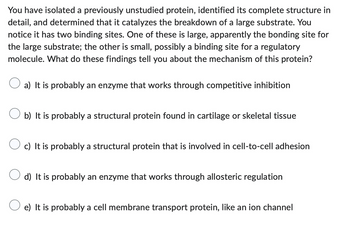
Transcribed Image Text:You have isolated a previously unstudied protein, identified its complete structure in
detail, and determined that it catalyzes the breakdown of a large substrate. You
notice it has two binding sites. One of these is large, apparently the bonding site for
the large substrate; the other is small, possibly a binding site for a regulatory
molecule. What do these findings tell you about the mechanism of this protein?
a) It is probably an enzyme that works through competitive inhibition
b) It is probably a structural protein found in cartilage or skeletal tissue
c) It is probably a structural protein that is involved in cell-to-cell adhesion
d) It is probably an enzyme that works through allosteric regulation
e) It is probably a cell membrane transport protein, like an ion channel

Transcribed Image Text:Recent technological advances have made it more feasible than ever to work out the
three-dimensional structure of proteins. There is intense interest in this research
field, called structural biology. Why?
a) Understanding structure should help us understand function
b) Understanding structure can help in the design of drugs that alter the
function of certain proteins
c) Solving a protein's 3-D structure can lead to a better understanding of how
the molecule works-for example, by identifying the active site and
determining if there are regulatory sites
d) All of the reasons described in the other answers apply
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- (5.5, 5.7 & 5.8) Classify the following reaction. Ba(NO3)2(aq) + K₂SO4(aq) --> O acid-base reaction O gas-forming reaction O decomposition reaction O precipitation reactionarrow_forwardter answ Aqueo us sulfurous acid (H2S03) was made by dissolving 0.200 L of sulfur dioxide gas at 19°C and 745 mm Hg in water to yield 500.0 mL of solutio n. The acid solution required 17.6 mL of so dium hydroxide solution to reach the titration end point. What was the molarity of the sodium hy droxide solution? M NaOH IID $4 7. COarrow_forwardBr MgBr (1 eq.), Et₂Oarrow_forward
- (a) 1.23 M sugar (C₁₂H₂O₁) solution (density of solution 1.12 g/mL) 1.09 201 (b) 0.849 M NaOH solution (density of solution 1.04 g/mL) m (c) 5.21 M NaHCO, solution (density of solution 1.19 g/mL) m Guided Sarrow_forward8:35 A moodle.nct.edu.om P Flag question Answer the following questions by using mole concept. (a) Calculate the molarity of a 105 g NazSO4 in 570 ml solution. [Atomic mass: Na=23 g/mol, S= 32 g/mol, O=16 g/mol, C=12 g/mol, H= 1 g/mol] (a1)no of moles of Na,SO4 (a2) molarity of solution (b) 29 g C6H12Og is dissolved in 210 g of water. Find out the molality of the solution. (b1) no of moles of CeH1206 (b2) molality of solution (c) How much volume of 1.9 M NaOH solution can be made by diluting 74 ml of 4 M NaOH. (d) Aspirin (CgHgO4) is a medication used to reduce pain, fever, or inflammation. Find out the mass of 3.5x1023 molecules of aspirin in gram. (d1) no of moles of Aspirin (d2) mass of aspirin Question 28 -> 11 •.. +arrow_forwardYou grab a soft drink bottle from your refrigerator. The contents are liquid and remain liquid even when shaken. You now remove the cover, and the liquid solidifies. Provide an explanation for this finding.arrow_forward
- More than 40% of the world's population has limited access to clean water. One proposed method for the production of clean water involves removing salt from seawater. Which technique would be used to separate the salt from the saltwater mixture?arrow_forwardIt keeps telling me I have the wrong answer, am I inputting it incorrectly??arrow_forwardSubstance A is a liquid at room temperature, dissolves substance B, and the resulting solution can conduct electricity. Substance C does not dissolve in substance A. Which of the following statements correctly identifies the substances? Substance A and B are ionic, and substance C is non-polar. Substance A is polar molecular, substance B and Substance C are ionic. Substance A is polar molecular, substance B is ionic, and Substance C is non- polar. All three substances are polar. Substance A is non-polar molecular, substance B is polar, and Substance C is polar.arrow_forward
- What volume of 0.185 M MgCl₂ must be added to 235 mL of 0.206 M KCl to produce a solution with a concentration of 0.250 M CI? (Ans. 86.2 mL) What volume of water must be added to 50.0 mL of 6.00 M HCl in order to reduce its molarity to 0.400 M? (Ans.700. mL)arrow_forwardPLEASE PROVIDE DETAILED SOLUTION AND GIVE THE EXPLANATION OF CORRECT AND INCORRECT OPTIONS....arrow_forward2 OH MeO OTBS MeO OTBS Me Me 3 HO Me 4arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY