
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Transcribed Image Text:111
Naming and Drawing Organic Molecules
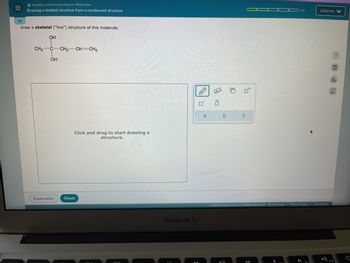
Drawing a skeletal structure from a condensed structure
Draw a skeletal ("line") structure of this molecule:
OH
CH3 C-CH2-CH=CH2
OH
Click and drag to start drawing a
structure.
Explanation
Check
алл
P
σ Ö
X
MacBook Air
G
P
2/5
Julianna
?
Ar
2024 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accessibility
A1
F12
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Name the Compound CH3 CH3 - CH – CH = CH2arrow_forwardC Bb Bl 01edu.co Maps ! 1 Q A L :0 F1 N 2 CH CH CH Ac Th M □ Dr Ak M: b H G 17 C SCUO Ne S 15 Bexb Ar Question 23 of 48 Provide the IUPAC name for the condensed structural formula shown here. CH₂ CH₂. CH3 CH CH₂ F2 W S X H #3 80 F3 E D C $ 4 F4 R F do L % 5 V 1- F5 H3C T H₂C G 6- neo eth hex F11 L + 11 [ Gra F12 G + } 1arrow_forwardPlease explain what type of isomer is this ?arrow_forward
- Pentane has several isomers. Which of the following condensed formulas is NOT an isomer of pentane? O CH3CH₂C (CH3)2 O CH2(C2H5)2 O CH3CH₂CH₂CH2CH3 O CH3C(CH3)3arrow_forwardDraw the condensed or skeletal structures for 2-pentanol OH CH3CH₂CH₂-CH-CH3 CH3 CH₂ CH₂ CH₂ CH₂-OH SH SHarrow_forward2. Determine which of the structures are isomers of each other and which are conformers of each other. c-c-c-C c-c-c- --- C-C-C-C C-C-C '' C-C-C-C سے ہے ہے ہے دل اليد <-٤-٤ C c- ؟-؟ Carrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY