
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
it is asking for the condensed form but we did not practice condensed form in class so i’m confused how it would look like for this compound
Transcribed Image Text:1 of 38 >
Macmillan Learning
(1)
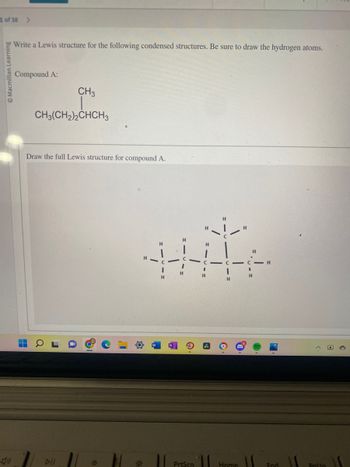
Write a Lewis structure for the following condensed structures. Be sure to draw the hydrogen atoms.
Compound A:
▬
7
CH3
CH3(CH2)2CHCH3
Draw the full Lewis structure for compound A.
DII
H
H
H
C
H
Prisen
H
H
B
Home
H
C-H
H
11
End
Palin
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Given that the condensed structure for GABA is H2N(CH2)3CO2H Find (A) the expanded structure and (B) the Line Structurearrow_forwardHow do you know if compounds have the same skeletal structure?arrow_forward19. SF, 20. BrF: 21. SiO; 22. NH.CIO: (indicate the alectron pair gecmatny and molecular shapa around sacha ion.)arrow_forward
- Draw the structures of the products of the neutralization reaction between methylamine and propanoic acid.arrow_forwardExamine the curved arrow below, and answer the question in the box. If the curved arrow creates a reasonable structure, draw the structure it creates. Show all lone pairs and non-zero formal charges. If the curved arrow does not create a reasonable structure leave the drawing space blank. Does the curved arrow create a reasonable structure? yes O no H.arrow_forwardDraw a Lewis structure for the molecule below, showing all lone pairs. You may abbreviate any methyl groups as CH,. CH,CH,OCH,CHCH, Click and drag to start drawing a structure.arrow_forward
- Please don't provide handwritten solution ....arrow_forwardExamine the following list of functional groups: alcohol, amine, carboxylic acid, carboxylic ester, and ketone. For a molecule having the molecular formula C5H10O, the two functional groups from the above list that CAN be in this molecule are (please explain your reason)arrow_forwardDraw the condensed structure of 3-chloro-4-methyloctane. Click anywhere to draw the first atom of your structure. toarrow_forward
- Draw the condensed structure of an isomer of this molecule: CH3-CH2 -CH2 CH3 Click anywhere to draw the first atom of your structure.arrow_forwardIn one of the two boxes below, draw a wedge and dashed wedge structure (picture) of CH3Cl that best illustrates the geometry about the central atom. In the other box, draw another picture of the model from a different angle (viewpoint).arrow_forwardFor each of the following molecules in the list: 1) List the total number of valence electrons and then draw the Lewis dot structure. 2) Use the kits to make a model of each molecule or ion. (A specific ball may not exist for the type of atom that you are looking for. If this happens, use a ball for which the number of holes matches the number of electron areas needed.) 3) Name the electron pair geometry. 4) Name the molecular shape. 5) Draw a 3-D sketch of the molecular shape and list the bond angles. 6) If the molecule contains covalent bonds, indicate if it is polar or nonpolar. 7) When necessary, draw a polar arrow for each bond. 8) If the molecule is polar, draw an arrow next to the 3-D sketch indicating the direction of the dipole moment. Molecular Lewis Dot AXE Type Electron on Central Pair Atom Molecular Ionic, polar, or nonpolar 3-D Sketch Formula Structure Shape with bond Geometry angles and dipole arrowarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY