
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:=
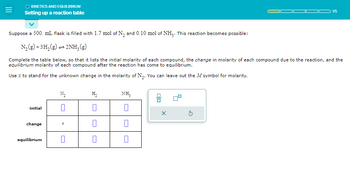
KINETICS AND EQUILIBRIUM
Setting up a reaction table
Suppose a 500. mL flask is filled with 1.7 mol of N₂ and 0.10 mol of NH3. This reaction becomes possible:
N₂(g) + 3H₂(g)2NH, (g)
Complete the table below, so that it lists the initial molarity of each compound, the change in molarity of each compound due to the reaction, and the
equilibrium molarity of each compound after the reaction has come to equilibrium.
Use x to stand for the unknown change in the molarity of N₂. You can leave out the M symbol for molarity.
initial
change
equilibrium
N₂
0
0
H₂
0
0
0
NH3
0
0
00
1/5
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Nitrogen dioxide is one of the many oxides of nitrogen (often collectively called "NOx") that are of interest to atmospheric chemistry. It can react with itself to form another form of NOx, dinitrogen tetroxide. A chemical engineer studying this reaction fills a 125 L tank with 45. mol of nitrogen dioxide gas. When the mixture has come to equilibrium he determines that it contains 12. mol of nitrogen dioxide gas. The engineer then adds another 11. mol of nitrogen dioxide, and allows the mixture to come to equilibrium again. Calculate the moles of dinitrogen tetroxide after equilibrium is reached the second time. Round your answer to 2 significant digits. | mol x10arrow_forwardNitrogen dioxide is one of the many oxides of nitrogen (often collectively called "Nox") that are of interest to atmospheric chemistry. It can react with itself to form another form of NOx, dinitrogen tetroxide. A chemical engineer studying this reaction fills a 75 L tank with 23. mol of nitrogen dioxide gas. When the mixture has come to equilibrium she determines that it contains 17.9 mol of nitrogen dioxide gas. The engineer then adds another 7.7 mol of nitrogen dioxide, and allows the mixture to come to equilibrium again. Calculate the moles of dinitrogen tetroxide after equilibrium is reached the second time. Round your answer to 2 significant digits.arrow_forwardWrite the equilibrium expression (Ke) for the reaction: PC1s(g) PC13(8) + C12(g)arrow_forward
- Suppose that the equilibrium constant for the chemical reaction has an equilibrium constant of 2.3 x 107. What is the equilibrium constant for the reverse reaction?arrow_forwardreaction K Br(aq) + AgNO3(aq) → KNO3(aq) + AgBr(s) CH₂OCH₂ (1) + 30₂(g) → 2CO₂(g) + 3H₂O(g) 2NaClO₂ (s) 2NaCl (s) + 30₂ (g) 2 HBr(aq) + NaOH(aq) → NaBr(aq) + H,O (1) 00000000000000 type of reaction (check all that apply) combination single replacement double replacement decomposition combination single replacement double replacement decomposition combination single replacement double replacement decomposition combination single replacement double replacement decomposition X 000 000 000 000 precipitation combustion acid-base precipitation combustion acid-base precipitation combustion acid-base precipitation combustion acid-base Śarrow_forward"Synthesis gas" is a mixture of carbon monoxide and water vapor. At high temperature synthesis gas will form carbon dioxide and hydrogen, and in fact this reaction is one of the ways hydrogen is made industrially. A chemical engineer studying this reaction fills a 75 L tank with 22. mol of carbon monoxide gas and 26. mol of water vapor. When the mixture has come to equilibrium he determines that it contains 13.5 mol of carbon monoxide gas, 17.5 mol of water vapor and 8.5 mol of hydrogen gas. The engineer then adds another 7.0 mol of water, and allows the mixture to come to equilibrium again. Calculate the moles of carbon dioxide after equilibrium is reached the second time. Round your answer to 2 significant digits. || mol x10arrow_forward
- Suppose a 250. mL flask is filled with 0.20 mol of N2 and 2.0 mol of NO. The following reaction becomes possible: N2(g) + O2(g) = 2NO(g) The equilibrium constant K for this reaction is 0.693 at the temperature of the flask. Calculate the equilibrium molarity of N2. Round your answer to two decimal places. Шмarrow_forwardSuppose a 250. mL flask is filled with 0.60 mol of N, and 0.70 mol of NO. This reaction becomes possible: N2(g) +0,(g) = 2NO(g) Complete the table below, so that it lists the initial molarity of each compound, the change in molarity of each compound due to the reaction, and the equilibrium molarity of each compound after the reaction has come to equilibrium. Use x to stand for the unknown change in the molarity of N,. You can leave out the M symbol for molarity. N, NO initial change equilibrium 미미arrow_forwardSuppose a 250. mL flask is filled with 0.30 mol of O, and 1.5 mol of NO. This reaction becomes possible: N, (g) +0,(g) = 2NO(g) Complete the table below, so that it lists the initial molarity of each compound, the change in molarity of each compound due to the reaction, and the equilibrium molarity of each compound after the reaction has come to equilibrium. Use x to stand for the unknown change in the molarity of N,. You can leave out the M symbol for molarity. alo Ar N2 O2 NO initial change equilibriumarrow_forward
- Suppose a 250. mL flask is filled with 1.9 mol of O, and 1.5 mol of NO. The following reaction becomes possible: N2 (g) +0,(g) =2NO(g) The equilibrium constant K for this reaction is 8.51 at the temperature of the flask. Calculate the equilibrium molarity of NO. Round your answer to two decimal places. OMarrow_forwardFor the reaction 2 NO2 (g) <=> N2O4 (g), K= 0.67. If [NO2] = 2.0 M, and the [N2O4] = 1.0 M , which of the following is true? The reaction is at equilibrium The reaction will move to the reactant side The reaction cannot reach equilibrium The reaction will move to the product sidearrow_forwardSuppose a 250. mL flask is filled with 0.90 mol of H, and 0.40 mol of NH,. This reaction becomes possible: N, (g) + 3H,(g) = 2NH, (g) Complete the table below, so that it lists the initial molarity of each compound, the change in molarity of each compound due to the reaction, and the equilibrium molarity of each compound after the reaction has come to equilibrium. Use x to stand for the unknown change in the molarity of N,. You can leave out the M symbol for molarity. N, H, NH, initial ? change equilibriumarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY