
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
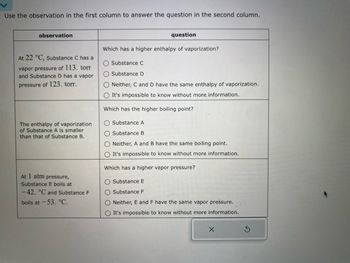
Transcribed Image Text:Use the observation in the first column to answer the question in the second column.
| Observation | Question |
|-----------------------------------------------------------------------------------------------|-----------------------------------------------------------------------|
| At 22 °C, Substance C has a vapor pressure of 113 torr and Substance D has a vapor pressure of 123 torr. | Which has a higher enthalpy of vaporization? |
| | ○ Substance C |
| | ○ Substance D |
| | ○ Neither, C and D have the same enthalpy of vaporization. |
| | ○ It's impossible to know without more information. |
| The enthalpy of vaporization of Substance A is smaller than that of Substance B. | Which has the higher boiling point? |
| | ○ Substance A |
| | ○ Substance B |
| | ○ Neither, A and B have the same boiling point. |
| | ○ It's impossible to know without more information. |
| At 1 atm pressure, Substance E boils at −42 °C and Substance F boils at −53 °C. | Which has a higher vapor pressure? |
| | ○ Substance E |
| | ○ Substance F |
| | ○ Neither, E and F have the same vapor pressure. |
| | ○ It's impossible to know without more information. |
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- REFER TO IMAGEarrow_forwardUse the observation in the first column to answer the question in the second column. observation question Which has a higher enthalpy of vaporization? At 1 atm pressure, Substance A Substance A boils at 84. °C and Substance B boils at Substance B 114. °C. Neither, A and B have the same enthalpy of vaporization. It's impossible to know without more information. Which has a higher boiling point? At -9 °C, substance E has Substance E a vapor pressure of 89. torr and Substance F has a vapor Substance F pressure of 59. torr. Neither, E and F have the same boiling point. It's impossible to know without more information. Which has the higher boiling point? Substance C The enthalpy of vaporization of Substance C is bigger than that of Substance D. Substance D Neither, C and D have the same boiling point. It's impossible to know without more information. O O O O O O O Oarrow_forwardActivity 1. Solve and answer the activity on a separate sheet and yourobservations on the diagrams presented. Solving Involving Heat and Change of State (Show Your Solution) 5. Acetic acid has a heat of fusion of 10.8 kJ/mol and a heat of vaporization of24.3 kJ/mol. What is the expected value for the heat of sublimation of aceticacid?arrow_forward
- The molar heat of vaporization of water at 25 °C is +43.9 kJ/mol. How many kilojoules of heat would be required to vaporize 1.01 g of water? This is the question I need help with. please can you guys respond as soon as possible.arrow_forwardThe vapor pressure of Substance X is measured at several temperatures: temperature vapor pressure 0 -60. °C -51. °C -42. °C 0.0132 atm 0.0393 atm 0.107 atm Use this information to calculate the enthalpy of vaporization of X. Round your answer to 2 significant digits. Be sure your answer contains a correct unit symbol. 0 ロ・ロ X μ 010 Śarrow_forwardUse the observation in the first column to answer the question in the second column. observation question At any temperature where both substances are liquid, which has the higher vapor pressure? O Substance E The enthalpy of vaporization of Substance E is smaller Substance F than that of Substance F. Neither, E and F have the same vapor pressure. It's impossible to know without more information. Which has a higher boiling point? At 71 °C, substance A has a Substance A vapor pressure of 108. torr and Substance B has a vapor Substance B pressure of 158. torr. Neither, A and B have the same boiling point. It's impossible to know without more information. Which has a higher enthalpy of vaporization? At 1 atm pressure, Substance C Substance C boils at 138. °C and Substance D Substance D boils at 171. °C. Neither, C and D have the same enthalpy of vaporization. It's impossible to know without more information.arrow_forward
- Use the observation in the first column to answer the question in the second column. observation question Which has the higher boiling point? Substance C The enthalpy of vaporization of Substance C is bigger than that of Substance D. Substance D Neither, C and D have the same boiling point. It's impossible to know without more information. Which has a higher enthalpy of vaporization? At 92 °C, substance E has a vapor pressure of Substance E 137. torr and Substance F Substance F has a vapor pressure of 97. torr. Neither, E and F have the same enthalpy of vaporization. It's impossible to know without more information. Which has a higher enthalpy of vaporization? At 1 atm pressure, Substance A Substance A boils at 146. °C and Substance B Substance B boils at 107. °C. Neither, A and B have the same enthalpy of vaporization. It's impossible to know without more information. O O O O O O O O O O Oarrow_forwardNeed help, please.arrow_forwardUse the observation in the first column to answer the question in the second column. observation The enthalpy of vaporization of Substance A is smaller than that of Substance B. At 1 atm pressure, Substance E boils at -18. °C and Substance F boils at -32. °C. At 68 °C, Substance C has a vapor pressure of 141. torr and Substance D has a vapor pressure of 101. torr. question At any temperature where both substances are liquid, which has the higher vapor pressure? Substance A O Substance B O Neither, A and B have the same vapor pressure. It's impossible to know without more information. Which has a higher vapor pressure? Substance E O Substance F ONeither, E and F have the same vapor pressure. O It's impossible to know without more information. Which has a higher boiling point? Substance C O Substance D O Neither, C and D have the same boiling point. O It's impossible to know without more information. Xarrow_forward
- Use the information provided to calculate the quantity of heat (in kJ) required to convert 59.22 g of substance A from an initial temperature of -8.998ºC to a final temperature of 53.2ºC. The molar mass of the substance is 31.20 g/mol. Melting Point of A = 1.110ºC Heat of fusion = 11.32 kJ/mol Boiling point of A = 109.9ºC Heat of vaporization = 86.91 kJ/mol Solid Liquid Gas Specific heat (J/gºC) 2.22 4.1 6.4arrow_forwardWhat takes more energy: raising water to its boiling point, or making it become water vapor? Why (mention molecular forces)?arrow_forwarda) Water can be sterilized by boiling. How much heat is required to heat 6 gallons of liquid water? (1 gallon=3.78 liters) from room temperature (77°F) to 212°F? b) If the heat of vaporization of water is 40.7 kJ/mol, how much heat would be required to convert the 6 gallons of water now at 212° to steam? Assume the density of water is 1 g/mL at 77°F.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY