
Chemistry: The Molecular Science
5th Edition
ISBN: 9781285199047
Author: John W. Moore, Conrad L. Stanitski
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please correct answer and don't used hand raiting
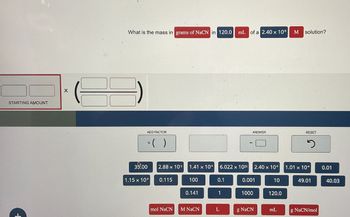
Transcribed Image Text:STARTING AMOUNT
X
What is the mass in grams of NaCN in 120.0
mL of a 2.40 x 10-5
M solution?
ADD FACTOR
* ( )
ANSWER
RESET
ว
35.00
2.88 x 103
1.41 x 10+
6.022 x 1023 2.40 x 10%
1.01 x 10
0.01
1.15 x 104
0.115
100
0.1
0.001
10
49.01
40.03
0.141
1
1000
120.0
mol NaCN M NaCN
L
g NaCN
mL
g NaCN/mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- What is the difference between (a) mass and density? (b) an extensive and an intensive property? (c) a solvent and a solution?arrow_forward150 140 130 120 110 100 NaNO3 90 80 70 60 NH&C KCI Naci 50 40 30 20 KCIO3. 10 Cez(SOA)3 0 10 20 30 40 50 60 70 80 90 100 Temperature ('C) EONX NH37 Grams of solutearrow_forward1.5 x 103 uL ------> mLarrow_forward
- Constants I Peric 1.00 g KCl in 95.0 mL of solution Express your answer with the appropriate units. µA M = Value Units Submit Request Answer Part B 1.00 g Na2CrO4 in 95.0 mL of solution Express your answer with the appropriate units. HẢ M = Value Units C.arrow_forward< STARTING AMOUNT X A brine solution is 3.50% NaCl by mass. Given that its density is 1.071 g/mL , determine the quantity of liters of solution that contains 5.00 moles of NaCl 5.00 ADD FACTOR 0.1 *( ) 3.50 10 35.45 Question 29 of 33 1.071 0.01 8.03 x 1022 1000 100 ANSWER 7.80 22.99 0.0156 L solution g NaCl/mL g solution mL solution kg solution g NaCl/g solution 58.44 6.022 x 1023 0.0780 moles of NaCl mol NaCl RESET 0.001 2 1 g NaCl 1.56arrow_forward1.Aarrow_forward
- What is 12.235g/1.01mLarrow_forwardView Assessment ← C Pri Ter Acc X 13 minutes remaining Question 1 blackboard.matc.edu/ultra/courses/_525703_1/outline/assessment/_1556 Blank 1 X Blank 2 Blank 3 All answers must have the correct units. Watch your significant digits! A 55.0 g sample of sugar (C12H22011) is dissolved in 170. g of water. a. What is the mass of the solute? Blank 1 g b. What is the mass of the solvent? Blank 2 g c. What is the mass of the solution? Blank 3 g d. What is the percentage by mass of this solution? (% m/m) Blank 4 % Blank 4 Add your answer Add your answer Add your answer Add your answer Courses Last saved 12:59:40 PM X Questions Filter (6) ▼ + K First < Previous Nextarrow_forwardCalculate the volume in milliliters of a 2.18M sodium chloride solution that contains 375. mmol of sodium chloride (NaCI) . Round your answer to 3 significant digits. OmlL ||mL x10 Submit Assignment Continue 2022 McGraw Hill LLC. AIl Rights Reserved. Terms of Use Privacy Center I Accessibility IMG-4492.jpg IMG-4482.jpg IMG-4481.jpg IMG-4480.jpg IMG-4493.jpg MacBook Air DD 80 F7 F8 F9 F10 F11 F1 F2 F3 F4 F5 F6 @ # % & * ( 2 3 4 5 6 7 8 %24arrow_forward
- 1. "Diffusion" sounds slow, but on the scale of a cell it is very fast. The average instantaneous velocity of a particle in solution is v= (kT/m)", where k = 1.38 X 101 g cm?/K sec², T= temperature in K, m = mass in g/molecule. A) Calculate the instantaneous velocity of a molecule of glucose and a molecule of myoglobin (a protein), at 37°C [molecular mass for glucose = 180 g/mol; molecular mass for myoglobin = 15,000 g/mol].arrow_forwardA student used 10 mL water instead of 30 mL for extraction of salt from mixture. How may this may this change the percentage of NaCI extractecd?arrow_forwardWhat is the solubility of KCI at 80C 150 140 KI 130 120 110 100 NANO3 90 80 70 60 NH4CI KCI NacCl 50 40 30 20 KCIÓ3 10 Ce2(SO4)3 0 10 20 30 40 50 60 70 80 90 100 Temperature (°C) DELL Grams of solute per 100 g H,0 NH37 EONXarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning