
Introduction to General, Organic and Biochemistry
11th Edition
ISBN: 9781285869759
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
For each reaction, redraw the reactants as skeletal structures then give the product
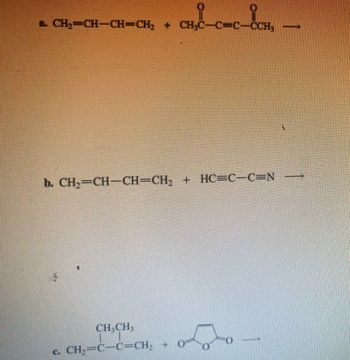
Transcribed Image Text:– CH₂–CH–CH=CH, + CH₂C_C=C_СCH,
b. CH2=CH—CH=CH, + HC=C—C=N
CH.CH.
c. CH-—C—C=CH-
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 3 images

Knowledge Booster
Similar questions
- From the data in Figure 4-12 and Table 4-1, estimate the percentages of molecules that have their substituents in an axial orientation for the following compounds: (a) Isopropylcyclohexane (b) Fluorocyclohexane (c) Cyclohexanecarbonitrile, C6H11CNarrow_forwardBr Hu Br 7 CI ECH H,C=CH 4 HC=CH HC=C Br HC=CH H2C=CH2 1arrow_forwarda CH,=CHCH,CHCH,CHCH, CH CH3 CH,CH, CH,C CCHCH,CH,arrow_forward
- Write the systematic name of each organic molecule: (10 HO—C—CH–CH2–CH–CH2–CH2−C CH3 I CH3 structure || HO—C—CH—CH,—OH CH3 CH3 CI-CH₂-CH-CH₂-C-OH name 0arrow_forwardH ₁ H 2 H :S: ||3 C=C-CH₂-S-C-N CH3 CH3arrow_forward60. Identify the type of each compound based on the function group it contains.arrow_forward
- O CH3-C-O-CH3 O || CH3-CH₂-C-OH C-OH 1. NaOH 2. H3O+ 1. SOCI₂ 2. CH3NH₂ CH3OH H+ cat.arrow_forwardGive an IUPAC name for each of the following molecules. Include the prefix cis- or trans- when appropriate. CI CI C=C a. Br Br H. CH, C=C CH b. Br CH,CH, C=C CH,CH, C. H. C=C] d. CH3arrow_forwardYEA CH3 CH C C-C-CH,CH, CH3 b. CH3 CH3 C. CH3 CH,CH3arrow_forward
- Write the systematic name of each organic molecule: CH3 structure -C−NH–CH2−CH2CH3 || H₂N-C-CH₂ -CH2–CH2–CH2–CH3 CH3 CH2–CH2–NH–C–CH2–CH3 name 11 0 0arrow_forwardWrite the systematic name of each organic molecule: O || CH3–CH2–CH2–CH2−C−N–CH2–CH2–CH3 H₂N CH3 CH₂- structure || || CH₂ CH₂ CH3 C-CH₂-CH3 NH C−NH–CH,—CH,—CH3 name 1 0arrow_forwardfull structural formula please for everythingarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
