
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please answer type in words don't image upload thank you.
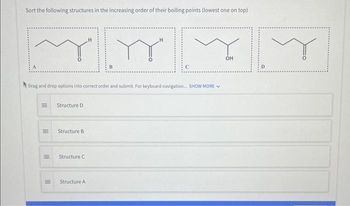
Transcribed Image Text:Sort the following structures in the increasing order of their boiling points (lowest one on top)
Drag and drop options into correct order and submit. For keyboard navigation.... SHOW MORE
|||
III
III
=
III
E
Structure D
Structure B
Structure C
Structure A
OH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 13 ← CO Biblio Manuels-fren.... CH Draw the curved arrows showing a proton transfer reaction, and draw the products of that proton transfer. Do not include the Li+ counterion, and lone pairs are not required in the products. + H-O-H Edit Drawing CMCbx bbwb + C .../1 11:11 PM 2024-02-09 0 AINarrow_forwardneht_805_Hydrates_1_2_1 (1) (Protected View) - Word (Unlicensed Product) rences Mailings Review View Help Foxit PDF Tell me what you want to do n contain viruses. Unless you need to edit, it's safer toay in Protected View. Enable Editing rour Office product is inactive. To use for free, sign in and use the Web version. Activate Use free at Office.com Part 3: Reversibility of hydration 1. Transfer a small amount of solid copper (II) sulfate pentahydrate, CUSO4-5H;O, that just fills the bottom of a clean, dry 150-mm (medium size) test tube. 2. Using a test tube holder, grasp the test tube containing the hydrate and heat over a Bunsen burner flame while holding the test tube at a 45° angle. 3. While heating, closely observe the solid and the inside wall of the test tube. Record your observations. 4. When the solid residue seems to be completely dehydrated, allow the test tube to cool completely. Then, add a few drops of laboratory water to Record your observations. solid in the test tube.…arrow_forwardView History Bookmarks People Tab Window Help ams, Amaya K - Outlo x B Chapter 5- CHEM-1110-941- X O Question 16 - Chapter 5 - Conr X + nheducation.com/ext/map/index.html?_con%=con&external_browser%3D0&launchUrl=https%253A%252F%252Flr Saved 3 attempts left Check my work Enter your answer in the provided box. A gas at 737 mmHg and 37°C occupies a volume of 9.56 L. Calculate its volume at STP. 116 MAR 664 17 18arrow_forward
- S outo Classes e myBackpack C Infinite Campus 9 New Tab File| /media/fuse/drivefs-a644cf067c63cc0d1217e47dfd368497/root/k.g_k.sch_ZG9taW5pY28ua2FudG9yQGFwc2sxMi5vcmc_Whiteboar. Q < * 1 /1 Good Day 03 Be O +2 AIC→I AbO, •3 Be Cla a) what is the molar mass? BeO , AlzOa b) How many mols of beryllium chleride are produced from 13 mols of BeO?(1-step) ) How many grams of Al,O, are 9 mol AICI,? (2-step) ) How many grams of Al2Og are produced Srom 100g BeO (3-step) e) Determine the limit ing reartan ti 13 7mol BeO ;13.7mol Al Cl, produced from 5) Determine excess reactant 4Sa BrO j 75q AICIS US VO 11:12 @ %23 2$ % 4 7 8 backsp W e y d h karrow_forwardExperiment 605_Hydrates_1_2_1 (1) (Protected View) - Word (Unlicensed Product) ces Mailings Review View Help Foxit PDF Tell me what you want to do contain viruses. Unless you need to edit, it's safer to stay in Protected View. Enable Editing ir Office product is inactive. To use for free, sign in and use the Web version. Post-lab Questions Activate Use free at Office.cc 1. Calculate the mass percent of water for the hydrate, LINO,-3H;O. 2. What will be the probable effect if you kept the crucible completely covered during the entire heating and cooling processes? Would your calculated percent water in the hydrate be high, low, or unaffected? Explain. 3. If 2.752 g sample of Ca(NO:); XH;O is heated to constant mass, the residue weighs 1.941 g. Determine the value of x and the formula of the hydrate.arrow_forwardBased on the results of your experiment, do you have hard or soft water? Does this match the data for your area? Briefly explain your answer. Hard Water Trial 1 Hard Water Trial 2 Hard Water Trial 3 Initial Syringe Reading 1.0ml 1.0ml 1.0ml Final Syringe Reading 0.88ml 0.84ml 0.85ml Volume of EDTA Consumed 0.12ml 0.16ml 0.15ml Water Hardness ppm CaCO3 24.024ppmCaCO3 32.032 ppmCaCO3 30.03 ppmCaCO3 Average ppm 28.70 ppmCaCO3 Tap Water Trial 1 Tap Water Trial 2 Tap Water Trial 3 Initial Syringe Reading 1.0ml 1.0ml 1.0ml Final Syringe Reading 0.65ml 0.68ml 0.67ml Volume of EDTA Consumed 0.35ml 0.32ml 0.33ml Water Hardness ppm CaCO3 70.07 ppmCaCO3 64.064 ppmCaCO3 66.066 ppmCaCO3 Average ppm 66.73 ppmCaCO3 Soft Water Trial 1 Soft Water Trial 2 Soft Water Trial 3 Initial Syringe Reading 1.0ml 1.0ml 1.0ml Final Syringe Reading…arrow_forward
- ques 2 part carrow_forwardActivity I. True or False Directions: Read and analyze the statements below. Write True if the statement is correct and False if it is not. 1. Sucrose is a disaccharide 2. Starch is composed of many glucose units 3. Fructose is also known as blood sugar 4. Keratin is easily dissolved in water 5. Proteins are made up of nucleotides _6. The iron group of hemoglobin is called a heme _7. A nucleotide has three parts: nitrogenous base, sugar, and phosphate group 8. DNA has a double helix structure 9. Triglyceride is a protein _10. Generally, unsaturated fatty acids remain solid at room temperature grouparrow_forwardrome File Edit View History Bookmarks Profiles Tab Window Help * Dementia Frien x 2 unread) - dte x Lobby | Top Hat x O ALEKS Watch Gilmore x A www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IvdWKW_BBZZ16tTytly4Fcfu6zOtOf8C Spotify Web Playe.. M Common Ethical D.. O THERMOCHEMISTRY Calculating kinetic energy km Calculate the kinetic energy of a 2.6 × 10" kg airplane moving at a speed of 0.32 Round your answer to 2 significant digits. Explanation Check MAR 14arrow_forward
- Can someone please helparrow_forwardrome File Edit View History Bookmarks Profiles Tab Window Help Watch Gilmore Girls x 4 Dementia Friend Co (1 unread) - dt882) Lobby A www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-IvdWKW_BBZZ16tTytly Spotify Web Playe... M Common Ethical D... O THERMOCHEMISTRY Calculating kinetic energy m Calculate the kinetic energy of a 67. g bullet moving at a speed of 499. Round your answer to 2 significant digits. Explagation Checkarrow_forwardWhat is the name of the following? Submit Answer Use the References to access important values if needed for this question. Try Another Version 10 item attempts remaining Previous Nextarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY