
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Can someone please help
Transcribed Image Text:11:29 1
t of a 2.54-cm rainfal
urse.html?courseld=16531331&OpenVellumHMAC-0ctcef792b8bd319204877fe01aed550#10001
Update :
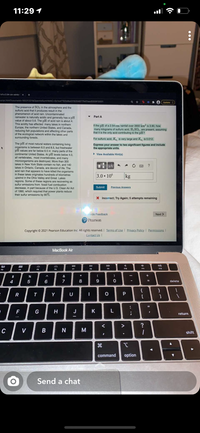
The presence of SO2 in the atmosphere and the
sulfuric acid that it produces result in the
phenomenon of acid rain. Uncontaminated
rainwater is naturally acidic and generally has a pH
value of about 5.6. The pH of acid rain is about 4.
This acidity has affected many lakes in northern
Europe, the northern United States, and Canada,
reducing fish populations and affecting other parts
of the ecological network within the lakes and
surrounding forests.
Part A
If the pH of a 2.54-cm rainfall over 3900 km? is 3.90, how
many kilograms of sulfuric acid, H2SO,, are present, assuming
that it is the only acid contributing to the pH?
For sulfuric acid, K is very large and K is 0.012.
The pH of most natural waters containing living
organisms is between 6.5 and 8.5, but freshwater
pH values are far below 6.5 in many parts of the
continental United States. At pH levels below 4.0,
all vertebrates, most invertebrates, and many
microorganisms are destroyed. More than 300
lakes in New York State contain no fish, and 140
lakes in Ontario, Canada, are devoid of life. The
acid rain that appears to have killed the organisms
in these lakes originates hundreds of kilometres
upwind in the Ohio Valley and Great Lakes
regions. Some of these regions are recovering as
sulfur emissions from fossil fuel combustion
decrease, in part because of the U.S. Clean Air Act
of 1990, which required that power plants reduce
their sulfur emissions by 80%.
Express your answer to two significant figures and include
the appropriate units.
> View Available Hint(s)
?
3.0 • 106
kg
Submit
Previous Answers
X Incorrect; Try Again; 5 attempts remaining
ovide Feedback
Next >
Pearson
Copyright © 2021 Pearson Education Inc. All rights reserved. I Terms of Use l Privacy Policy Permissions I
Contact Us I
MacBook Air
DD
80
888
F7
19
F10
F12
F3
FS
$
&
7
8
9
4
%3D
delete
{
R
Y
U
%3D
F
G
H
K
L
return
?
V
N
M
shift
command
option
Send a chat
V
B
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In a chemical equation, the ___________________ indicates that a chemical change occurred. what is the vocab?arrow_forward50 mL of stock solution were taken and added to flask. Then 50 mL of Di water were added to the flask. This is solution 1. Then 50mL of solution 1 were taken and added to a flask and 50 mL of Di water were added to the flask. This is solution 2. Then 50mL of solution 2 were taken and added to a flask and 50 mL of Di water were added to the flask. This is solution 3. Then 50mL of solution 3 were taken and added to a flask and 50 mL of Di water were added to the flask. This is solution 4. Find the concetrations of each solution. Information of stock solution - molar mass- 534.3g/mole 0.587g in MilliQarrow_forwardIn a 0.32 mM aqueous solution of trimethylacetic acid (C4H,CO₂H), what is the percentage of trimethylacetic acid that is dissociated? You can find some data that is useful for solving this problem in the ALEKS Data resource. Round your answer to 2 significant digits. % 0 x10arrow_forward
- When you are calculating water hardness, all the compounds in the water are considered to be -------- ? Select one: Limestone CaCO3 Hydrates MgCO3arrow_forwardFind the Salinity based on the grapharrow_forwardSolution 1 Solution 2 Solution 3 Solution 4 Solution 5 Concentration iron(III) nitrate 0.00200 0.00200 0.00200 0.00200 0.00200 [Fe(NO3)3] (M) Concentration potassium thiocyanate 0.00200 0.00200 0.00200 0.00200 0.00200 [KSCN] (M) Volume Fe(NO3)3 (mL) 5.00 5.00 5.00 5.00 5.00 Volume KSCN (mL) 5.00 4.00 3.00 2.00 1.00 Volume DI water (mL) 0.00 1.00 2.00 3.00 4.00 Initial concentration [Fe3+] (M) 0.00100 0.00100 0.00100 0.00100 0.00100 Initial concentration [SCN] (M) 0.00100 0.000800 0.000600 0.000400 0.000200 Absorbance 0.269 0.192 0.154 0.104 0.052 Equilibrium [FeSCN2+] (M) 0.000198 0.000141 0.000113 0.0000765 0.000038 Equilibrium constant Kc Average Kcarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY