
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
need help solving 13 and 14
Transcribed Image Text:https://assignment.itslearning.co X
L Labster Direct | Labster
+
com/mvc/Attachment/Get?Fileld%3DAQrEBRhlaUiTSbbBtxFh7%2f5rORZBT46%2fKssbODSNdUvgYDhmo
o itslearning v
P Search (Alt + Q)
References
Review
View
Help
Editing v
-11
A^
A
BIU
ev Av Ao
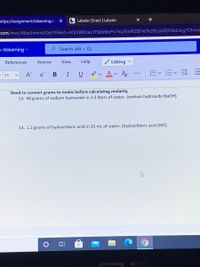
Need to convert grams to moles before calculating molarity
13. 98 grams of sodium hydroxide in 2.2 liters of water. (sodium hydroxide NaOH)
14. 1.2 grams of hydrochloric acid in 25 mL of water. (hydrochloric acid (HCI)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Masses of compounds used and observations NaCI CuSO4-5H20 Bright blue crystaline solids (NH4)2Fe(SO4)2 pale, Blue-green crystalline solid colorless Crystals Appearance of solid Mass of vial and 12.8364 12.7398 12.9445 compound (g) Mass of emptied vial (g) 11.6023 11.4888 11.9221 Mass of compound used (g) 1.2341. 1.2510. 1.0224 Volume of prepared solution = Type response here. Mass of sodium chloride used = Type response here.arrow_forwardHow did you get 2.69 x 10-12 ? When I did the caculation I got -5.16 x 10-22arrow_forwardNHẠNO3 MgSO4 [1] Mass of salt (g) 1.968 1.981 [2] Volume of DI water (mL) 50.0 50.0 0.039 0.0396 Mass of DI water (g) [3] Temperature of DI water (°C) 22.5 22.5 [4] Temperature of mixture after dissolution (°C) 19.5 27.1 3 4.6 Temperature difference (°C) [5] Total mass in reaction (g) [6] Enthalpy of solution AHsolution (cal/mol)arrow_forward
- A standard crank ice cream maker uses a mixture of ice water and salt to cool ice cream mix to the point of freezing. If ice cream freezes at 27 degrees Fahrenheit, what is the minimum concentration of salt needed to make ice cream?arrow_forwardЕ. NaCN Br Acetonearrow_forwardplease help me find the mmol for this one. Name Molecular Weight Amount Used mmol Equivalents Density Acetonitrile 41.04 3mL .786g/mLarrow_forward
- A oxygen with one single bond and three lone pairs is a neutral oxygen an oxonium ion with formal charge -1 an oxyanion ion with formal charge -1 an oxyanion with formal charge +1 an oxonium ion with formal charge +1arrow_forwardFill in all these boxes with correct units. dont fg to put units for each # put downarrow_forwardChemistry HW need help, how to do these caculation and answer these questions?arrow_forward
- Newly produced beer must be checked to see if it meets predetermined standards. The percentage of alcohol is determined by distillation. A set of measurements is listed forone batch.Sample Volume of beer (L) Ethanol distilled (mL)1 8.00 3922 10.00 5003 9.00 4594 9.00 444 What is the average percentage by volume of ethanol for this batch?arrow_forwardGive correct answer to all parts?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY