
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:View
History
Bookmarks
People
Tab
Window
Help
ams, Amaya K - Outlo x
B Chapter 5 - CHEM-1110-941 -
6 Question 15 - Chapter 5 - Coni X
heducation.com/ext/map/index.html?_con=con&external_browser=D0&launchUrl=https%253A%252F%252Flms.mhedu
Saved
3 attempts left
Check my work
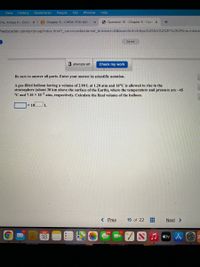
Be sure to answer all parts. Enter your answer in scientific notation.
A gas-filled balloon having a volume of 2.90 L at 1.20 atm and 16°C is allowed to rise to the
stratosphere (about 30 km above the surface of the Earth), where the temperature and pressure are –45
°C and 7.10 x 10¬3 atm, respectively. Calculate the final volume of the balloon.
x 10
L
< Prev
15 of 22
Next >
117
MAR
664
17
18
J átv A O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A hot air balloon has an initial volume of 1.80×106 L at a temperature of 25.0°C. What temperature must the air be heated to to expand the balloon's volume to 2.50×106 L at constant pressure.Express your answer to three significant figures.T2 = °Carrow_forwardSubject:arrow_forwardOzone molecules in the stratosphere absorb much of the harmful radiation from the sun. How many ozone molecules are present in 1.00 L of air under the stratospheric ozone conditions of 249 K temperature and 1.51 x 10 -3 atm pressure? Be sure your answer has the correct number of significant digits. molecules X S ∞o E Olo Ararrow_forward
- The Haber Process synthesizes ammonia at elevated temperatures and pressures. Suppose you combine 1580 L of nitrogen gas and 4240 L of hydrogen gas at STP, heat the mixture to run the reaction, then separate the ammonia from the reaction mixture. What volume of reactant, measured at STP, is left over? Assume the reaction goes to completion. N₂ (g) + 3 H₂ (g) → 2 NH₃ (g)arrow_forwardCalculate the pressure to which this volume of air must be compressed in order to fit into the air tank. Write your answer in atmospheres. Be sure your answer has the correct number of significant digits.arrow_forward2. Sodium sulfide (0,1159 g) and excess hydrochloric acid were reacted together to form 35.75 mL of an unknown gas. The gas was collected over water when the atmospheric pressure was measured to be 767 mmHg. The temperature of the water was 23.0°C. Determine the molar mass, as well as identity, of the gas. Hint: You will need to reference your textbook for the vapor pressure of water.arrow_forward
- A reaction between liquid reactants takes place at −17.0°C in a sealed, evacuated vessel with a measured volume of 35.0L . Measurements show that the reaction produced 39.g of dinitrogen difluoride gas. Calculate the pressure of dinitrogen difluoride gas in the reaction vessel after the reaction. You may ignore the volume of the liquid reactants. Be sure your answer has the correct number of significant digits. pressure: atmarrow_forwardA quantity of helium gas is originally held under a pressure of 145 kPa at 18°C. If the volume of the container is tripled, and the temperature is increased to 30°C, what is the resulting pressure in kPa? (Hint, can you make up a volume?) H 322181 Li Na Mg Rb Be Sr Cs Ba Fr 22 Ca Sc Ti Ra Raden Lat 94.5 kPa 46.4 kPa 378.3 kPa 453.9 kPa 50.3 kPa MD 45 83-103 482.2 kPa Zr Hf Atvice Ac Th Series 23 VO Vieta VID 24 2 Ta W Tete 104 105 Rf Db Sg Cr Mn Periodic Table of the Elements VID 75 41. 34445 Nb Mo Tc Ru Rh Pd Ag Cd Abaline Tra Saber 26 27 28 29 30 Fe Co Ni Cu Zn Re Os Symbol S 13 13 FIA B Nawww Al Ga Halagan In La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho D IVA 70 Pt Au Hg Tl Pb B Mar 102108, 109 114 Bh Hs Mt Ds Rg Cn Uut "FI 590 U Np Pu Am Cm Bk Cf Es Sn Sb 14 15 16 17 18 Si CI Ar La 16 3514. Ge As Se Br Kr By 17 VIA 18 VELA He Hek 4000 Ne 62 63 64 SA Te I Xe 16 At Rn Fate Bi Po Uup "Lv Uus Uuo Er Tm Yb Lu C Henan 100 100 Fm Md No Larrow_forwardA sample of methane gas collected at a pressure of 438 mm Hg and a temperature of 292 K has a mass of 11.2 grams. The volume of the sample is____L.arrow_forward
- According to Dalton, the pressure exerted by a gas inside a container is proportional to the number of molecules of that gas. If 4.00 x 1023 molecules of N2 and 2.00 x 1023 molecules of O2 are mixed in a container they exert a total pressure of 1686 torr. What portion of that pressure (in torr units) is exerted by the N2? Report answer to four significant figures in decimal notation. _____________________ torr, What portion of that pressure (in torr units) is exerted by the O2? Report answer to four significant figures in decimal notation. ____________________ torrarrow_forwardA balloon at 30.0 °C has a volume of 222 mL. If the temperature is increased to 63.1 °C and the pressure remains constant, what will the new volume be, in ml?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY