
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
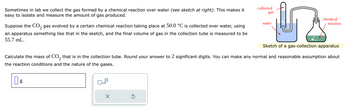
Transcribed Image Text:Sometimes in lab we collect the gas formed by a chemical reaction over water (see sketch at right). This makes it
easy to isolate and measure the amount of gas produced.
Suppose the CO₂ gas evolved by a certain chemical reaction taking place at 50.0 °C is collected over water, using
an apparatus something like that in the sketch, and the final volume of gas in the collection tube is measured to be
55.7 mL.
g
x10
057
Sketch of a gas-collection apparatus
x
collected
gas
Calculate the mass of CO₂ that is in the collection tube. Round your answer to 2 significant digits. You can make any normal and reasonable assumption about
the reaction conditions and the nature of the gases.
water
chemical
reaction
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Sometimes in lab we collect the gas formed by a chemical reaction over water (see sketch at right). This makes it easy to isolate and measure the amount of gas produced. Suppose the CO₂ gas evolved by a certain chemical reaction taking place at 50.0 °C is collected over water, using an apparatus something like that in the sketch, and the final volume of gas in the collection tube is measured to be 103. mL. g 0 collected gas X water ال Calculate the mass of CO₂ that is in the collection tube. Round your answer to 2 significant digits. You can make any normal and reasonable assumption about the reaction conditions and the nature of the gases. chemical reaction Sketch of a gas-collection apparatusarrow_forwardSometimes in lab we collect the gas formed by a chemical reaction over water (see sketch at right). This makes it easy to isolate and measure the amount of gas produced. Suppose the O₂ gas evolved by a certain chemical reaction taking place at 30.0 °C is collected over water, using an apparatus something like that in the sketch, and the final volume of gas in the collection tube is measured to be 114. mL. g x10 X collected Ś gas Sketch of a gas-collection apparatus Calculate the mass of O₂ that is in the collection tube. Round your answer to 2 significant digits. You can make any normal and reasonable assumption about the 2 reaction conditions and the nature of the gases. water IS chemical reactionarrow_forwardSometimes in lab we collect the gas formed by a chemical reaction over water (see sketch at right). This makes it easy to isolate and measure the amount of gas produced. Suppose the CO₂ gas evolved by a certain chemical reaction taking place at 40.0 °C is collected over water, using an apparatus something like that in the sketch, and the final volume of gas in the collection tube is measured to be 87.0 mL. collected gas X water chemical reaction Sketch of a gas-collection apparatus Calculate the mass of CO₂ that is in the collection tube. Round your answer to 2 significant digits. You can make any normal and reasonable assumption about the reaction conditions and the nature of the gases. BEET Ar Barrow_forward
- Sometimes in lab we collect the gas formed by a chemical reaction over water (see sketch at right). This makes it easy to isolate and measure the amount of gas produced. Suppose the O2 gas evolved by a certain chemical reaction taking place at 50.0°C is collected over water, using an apparatus something like that in the sketch, and the final volume of gas in the collection tube is measured to be 76.9mL. Sketch of a gas-collection apparatus Calculate the mass of O2 that is in the collection tube. Round your answer to 2 significant digits. You can make any normal and reasonable assumption about the reaction conditions and the nature of the gases. = garrow_forwardetermine the total volume of all gases formed when 75.0 mL an explosive (C3H5(NO3)3 , d = 1.60 g/mL, molar mass = 227.10 g/mol) reacts according to the following reaction. Assume room temperature and pressure conditions. 4 C3H5(NO3)3(l) → 6 N2(g) + O2(g) + 12 CO2(g) + 10 H2O(g)arrow_forwarda 2.802g sample of an unknown pure metal (M) is added to a 0.2 L solution of 2 mol/L H2SO4(aq). The metal reacts with the acid until the metal is completely gone to form M3+(aq) ion in the solution and produces hydrogen gas that is collected. The hydrogen gas that is collected over water that is isolated from the reaction has a volume of 0.82 L at 25 degrees Celsius and a total pressure of 704.0 mmHg. How many moles of H2(g) are produced? Write a balanced equation for the reaction of M with H2SO4(aq). What is the identity of metal M?arrow_forward
- Sometimes in lab we collect the gas formed by a chemical reaction over water (see sketch at right). This makes it easy to isolate and measure the amount of gas produced. Suppose the CO₂ gas evolved by a certain chemical reaction taking place at 55.0 °C is collected over water, using an apparatus something like that in the sketch, and the final volume of gas in the collection tube is measured to be 129. mL. g x10 177 Sketch of a gas-collection apparatus x collected gas Calculate the mass of CO₂ that is in the collection tube. Round your answer to 2 significant digits. You can make any normal and reasonable assumption about the reaction conditions and the nature of the gases. water chemical reactionarrow_forwardSometimes in lab we collect the gas formed by a chemical reaction over water (see sketch at right). This makes it easy to isolate and measure the amount of gas produced. Suppose the O2 gas evolved by a certain chemical reaction taking place at 35.0°C is collected over water, using an apparatus something like that in the sketch, and the final volume of gas in the collection tube is measured to be 48.7mL. Sketch of a gas-collection apparatus Calculate the mass of O2 that is in the collection tube. Round your answer to 2 significant digits. You can make any normal and reasonable assumption about the reaction conditions and the nature of the gases. = garrow_forwardSometimes in Io we collect the gas formed by a chemical reaction over water (see sketch at right). This makes it easy to isolate and measure the amount of gas produced. collected gas Suppose the O, gas evolved by a certain chemical reaction taking place at 30.0 °C is collected over water, using chemical reaction water an apparatus something like that in the sketch, and the final volume of gas in the collection tube is measured to be 44.8 mL. Sketch of a gas-collection apparatus ab Calculate the mass of O, that is in the collection tube. Round your answer to 2 significant digits. You can make any normal and reasonable assumption about the reaction conditions and the nature of the gases. Explanation Check O 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use I Privacy Center | Accessibility 6:20 AM N 35°F Mostly cloudy ENG 1/23/2022 Pll 26 506 F90 T. 4. R] T Y D Farrow_forward
- Sometimes in lab we collect the gas formed by a chemical reaction over water (see sketch at right). This makes it easy to isolate and measure the amount of gas produced. collected gas chemical reaction water Suppose the CÓ gas evolved by a certain chemical reaction taking place at 30.0 °C is collected over water, using an apparatus something like that in the sketch, and the final volume of gas in the collection tube is measured to be 131. mL. Sketch of a gas-collection apparatus alo Calculate the mass of CO that is in the collection tube. Round your answer to 2 significant digits. You can make any normal and reasonable assumption about the reaction conditions and the nature of the gases. Explanation Check O 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center| Acce: 12:05 AM ENG 42°F Cloudy 1/22/202 DELLarrow_forward#14arrow_forwardAccording to Dalton, the pressure exerted by a gas inside a container is proportional to the number of molecules of that gas. If 4.00 x 1023 molecules of N2 and 2.00 x 1023 molecules of O2 are mixed in a container they exert a total pressure of 1686 torr. What portion of that pressure (in torr units) is exerted by the N2? Report answer to four significant figures in decimal notation. _____________________ torr, What portion of that pressure (in torr units) is exerted by the O2? Report answer to four significant figures in decimal notation. ____________________ torrarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY