
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:**Transcription of Educational Content:**
A reaction between liquid reactants takes place at 12.0 °C in a sealed, evacuated vessel with a measured volume of 40.0 L. Measurements show that the reaction produced 39. g of sulfur hexafluoride gas.
Calculate the pressure of sulfur hexafluoride gas in the reaction vessel after the reaction. You may ignore the volume of the liquid reactants. Be sure your answer has the correct number of significant digits.
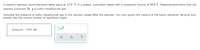
**Diagram Explanation:**
The diagram consists of a box labeled "pressure," containing the value "0.0002 atm." Under the box, there are icons indicating options related to interaction with the content, such as resetting or seeking help, but these are not related to the calculation.
This setup serves as a placeholder for entering or displaying the calculated pressure of the sulfur hexafluoride gas in the educational context.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A gas cylinder that contains argon has an internal volume of 43.8 L. A pressure gauge attached to the cylinder reads 1890 psi (1 atm = 14.7 psi) at 22.3 °C. What mass of argon is contained in the cylinder? mass = garrow_forwardA cylinder is filled with 10.0 L of gas and a piston is put into it. The initial pressure of the gas is measured to be 151. kPa. The piston is now pulled up, expanding the gas, until the gas has a final volume of 67.0 L. Calculate the final pressure of the gas. Round your answer to 3 significant digits. gas piston cylinderarrow_forwardA reaction between liquid reactants takes place at 30.0 °C in a sealed, evacuated vessel with a measured volume of 30.0 L. Measurements show that the reaction produced 17. g of dinitrogen monoxide gas. Calculate the pressure of dinitrogen monoxide gas in the reaction vessel after the reaction. You may ignore the volume of the liquid reactants. Round your answer to 2 significant digits. atm pressure:at x10 X Sarrow_forward
- A reaction between liquid reactants takes place at 29.0 °C in a sealed, evacuated vessel with a measured volume of 5.0 L. Measurements show that the reaction produced 49. g of sulfur hexafluoride gas. Calculate the pressure of sulfur hexafluoride gas in the reaction vessel after the reaction. You may ignore the volume of the liquid reactants. Be sure your answer has the correct number of significant digits. pressure: atm ?arrow_forwardA cylinder is filled with 10.0 L of gas and a piston is put into it. The initial pressure of the gas is measured to be 203. kPa. The piston is now pushed down, compressing the gas, until the gas has a final volume of 2.40 L. Calculate the final pressure of the gas. Round your answer to 3 significant digits. piston cylinder gasarrow_forward3 Enter your answer in the provided box.A sample of sulfur hexafluoride gas occupies 7.69 L at 218°C. Assuming that the pressure remains constant, what temperature (in °C) is needed to reduce the volume to 2.89 L? Report your answer to the proper number of significant figures.°Carrow_forward
- The barometric pressure is 750.8 torr. The water vapor pressure at the temperature at which the experiment is conducted is 21.711 torr. Determine the partial pressure of the gas in torr. Give your answer to the correct number of significant figures.arrow_forwardA marine biologist is preparing a deep-sea submersible for a dive. The sub stores breathing air under high pressure in a spherical air tank that measures 66.0 cm wide. The biologist estimates she will need 6800. L of air for the dive. Calculate the pressure to which this volume of air must be compressed in order to fit into the air tank. Write your answer in atmospheres. Round your answer to 3 significant digits. atm x10arrow_forwardSubject:arrow_forward
- Calculate the pressure to which this volume of air must be compressed in order to fit into the air tank. Write your answer in atmospheres. Be sure your answer has the correct number of significant digits.arrow_forwardA gas occupying a volume of 622.0 mL at a pressure of 0.860 atm is allowed to expand at constant temperature until its pressure reaches 0.547 atm. What is its final volume? Be sure your answer has the correct number of significant digits. mL Xarrow_forwardA reaction between liquid reactants takes place at −17.0°C in a sealed, evacuated vessel with a measured volume of 35.0L . Measurements show that the reaction produced 39.g of dinitrogen difluoride gas. Calculate the pressure of dinitrogen difluoride gas in the reaction vessel after the reaction. You may ignore the volume of the liquid reactants. Be sure your answer has the correct number of significant digits. pressure: atmarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY