
Chemistry for Engineering Students
3rd Edition
ISBN: 9781285199023
Author: Lawrence S. Brown, Tom Holme
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
From part a : answer the question

Transcribed Image Text:Questions
1. Give two qualitative observations for each
sample upon closer examination of the
respective photos.
2. Sample Four was the only sample that
contained an odour. What does this suggest
about the ease with which the particles can
leave the solid with respect to the strength
of the intermolecular forces of attraction
between the particles? (
3. Which sample has mobie electrons for
which to conduct electricity?
4. Sample Four was the only one that was
soluble in a nonpolar solvent. What does
this suggest about the polarity of the
molecule in Sample Four?
5. Sample One was soluble in water and in its
molten state conducted electricity, but in its
solid state it did not conduct electricity.
Electrons only flow in solids to produce
current electricity. What types of particles
does the experimental evidence point to the
makeup of this sample? (.
6. What evidence is there to support the
formation of ionic or molecular liquids of
nonpolar crystals?
7. What could you deduce about the strength
of the forces of attraction between the
particles, in Sample 3, compared to the
other samples? (1 mark)
8. Give 3 more physical tests to help you in
your analysis of these samples and their
subsequent identification.
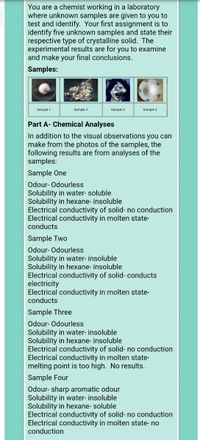
Transcribed Image Text:You are a chemist working in a laboratory
where unknown samples are given to you to
test and identify. Your first assignment is to
identify five unknown samples and state their
respective type of crystalline solid. The
experimental results are for you to examine
and make your final conclusions.
Samples:
Sample 1
Sample 2
Sample 3
Sample 4
Part A- Chemical Analyses
In addition to the visual observations you can
make from the photos of the samples, the
following results are from analyses of the
samples:
Sample One
Odour- Odourless
Solubility in water- soluble
Solubility in hexane- insoluble
Electrical conductivity of solid- no conduction
Electrical conductivity in molten state-
conducts
Sample Two
Odour- Odourless
Solubility in water- insoluble
Solubility in hexane- insoluble
Electrical conductivity of solid- conducts
electricity
Electrical conductivity in molten state-
conducts
Sample Three
Odour- Odourless
Solubility in water- insoluble
Solubility in hexane- insoluble
Electrical conductivity of solid- no conduction
Electrical conductivity in molten state-
melting point is too high. No results.
Sample Four
Odour- sharp aromatic odour
Solubility in water- insoluble
Solubility in hexane- soluble
Electrical conductivity of solid- no conduction
Electrical conductivity in molten state- no
conduction
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Elemental carbon has one gas phase, one liquid phase, and two different solid phases, as shown in the phase diagram: (a) On the phase diagram, label the gas and liquid regions. (b) Graphite is the most stable phase of carbon at normal conditions. On the phase diagram, label the graphite phase. (c) If graphite at normal conditions is heated to 2500 K while the pressure is increased to 1010 Pa, it is converted into diamond. Label the diamond phase. (d) Circle each triple point on the phase diagram. (e) In what phase does carbon exist at 5000 K and 108 Pa? (f) If the temperature of a sample of carbon increases from 3000 K to 5000 K at a constant pressure of 106 Pa, which phase transition occurs, if any?arrow_forwardIn terms of their bulk properties, how do liquids and solids differ? How are they similar?arrow_forwardThe shape of the meniscus of water in a glass tube is different from that of mercury in a glass tube. Why?arrow_forward
- Identify two common observations indicating some solids, such as dry ice and mothballs, have vapor pressures sufficient to sublime?arrow_forwardSubstance B is hard, does not conduct electricity, and melts at 1200 C. Substance B is likely a(n): (a) ionic solid (b) metallic solid (c) molecular solid (d) covalent network solidarrow_forwardThe amount of heat required to melt 2 lbs of ice is twice the amount of heat required to melt 1 lb of ice. Is this observation a macroscopic or microscopic description of chemical behavior? Explain your answer.arrow_forward
- Temperature Determine which temperature is higher, 110 °C or 180 °F. Determine which temperature is lower, 36 °C or 100 °F. The melting point of gallium is 29.8 °C. If you hold a sample of gallium in your hand, will it melt? Explain your answer.arrow_forwardWhat types of forces exist between the individual particles in an ionic solid? Are these threes relatively strong or relatively weak?arrow_forwardFour shiny solids are labeled A, B, C, and D. Given the following information about the solids, deduce the identity of A, B, C, and D. (1) The solids are a graphite rod, a silver bar, a lump of fool's gold (iron sulfide), and iodine crystals. (2) B, C, and D are insoluble in water. A is slightly soluble. (3) Only C can be hammered into a sheet. (4) C and D conduct electricity as solids; B conducts when melted; A does not conduct as a solid, melted, or dissolved in water.arrow_forward
- Two different pure solids are melted, and one exhibits a melting point of 138 oC, while the other exhibits a melting range of 135-147 oC. Explain the difference between the two.arrow_forwardA solid will melt to its liquid phase if enough heat is applied. A udy True O Search O Falsearrow_forwardIntroductionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning