
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
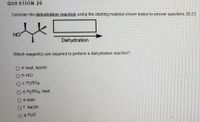
Transcribed Image Text:QUESTION 20
Consider the dehydration reaction using the starting material shown below to answer questions 20-23.
HO
Dehydration
Which reagent(s) are required to perform a dehydration reaction?
a. heat, NaOH
O b. HCI
OC. H2SO4
O d. H2SO4, heat
O e. NaH
O f. NaOH
Og. H20
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Need both!!!!arrow_forwardQuestion 14 For the following reaction, select the correct product. C. D. O B 8888arrow_forward1. The following molecule contains the functional groups A. secondary amine, secondary alcohol, ketone, carboxylic ester B. amide, primary alcohol, aldehyde, carboxylic acid C. secondary amine, two secondary alcohols, and two ketones D. primary amine, secondary alcohol, ketone, and carboxylic acid The following molecule contains the functional groups 2. A. primary alcohol and primary amine C. secondary alcohol and primary amine 3. A. alkene and secondary alcohol C. ketone and seconary alcohol The following molecule contains the functional groups B. ether and ketone D. ether and alkene 4. The following molecule A. primary amine and ketone C. secondary amine and alkyne NH₂ contains the functional groups B. secondary amine and alkene D. secondary amine and ketone ОН OH B. secondary alcohol and secondary amine D. primary alcohol and secondary amine NH₂ OHarrow_forward
- 11. Which of the following would be more stable than gasoline? a. Natural gas b. Kerosene C. Diesel d. Waxarrow_forward28. The formula for methyl ethanoate is which of the following? a. b. H3C- H3C-CH-CH3 OH -CH₂-CH3 C. d. H3C-CH₂-C-CH3 H3C- -0-CH3arrow_forwardQUESTION 7 Explain why aldehydes are water soluble. SELECT all that applies. There may be multiple answers. OA. Both aldehydes and water are polar and have a strong attraction between each other. O B. Both aldehydes and water are polar and can form dipole-dipole interaction with water OC. Aldehyde is less dense that water OD. Aldehyde can form hydrogen bonds with water O E. Aldehyde dissolves in water because it has a high medium boiling pointarrow_forward
- which of the folowing molecles will NOT be soluble in water? a. 2-butanol b. butanoic acid c. 2-butanone d. butyl amine e. 2-butynearrow_forwardWhich of the following can hydrogen bond with water? A. all of the above B. Carbon dioxide C. Methane D. Acetic acidarrow_forwardThis functional group is known as a(n) Select one: a. carboxyl b. hydroxyl c. ester. d. acetal e. carbonylarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY