
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
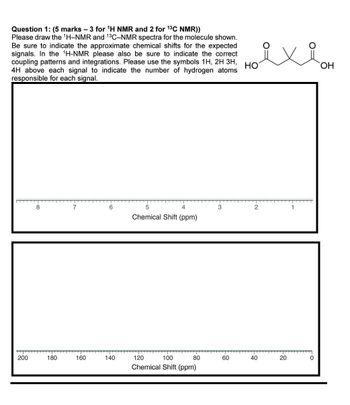
Transcribed Image Text:Question 1: (5 marks - 3 for 1H NMR and 2 for 13C NMR))
Please draw the 1H-NMR and 13C-NMR spectra for the molecule shown.
Be sure to indicate the approximate chemical shifts for the expected
signals. In the 1H-NMR please also be sure to indicate the correct
coupling patterns and integrations. Please use the symbols 1H, 2H 3H,
4H above each signal to indicate the number of hydrogen atoms
responsible for each signal.
HO
8
7
6
5
4
3
2
Chemical Shift (ppm)
200
180
160
140
120
100
80
60
40
10
Chemical Shift (ppm)
20
OH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 4 images

Knowledge Booster
Similar questions
- 3-Bromo-1-phenyl-1-propene shows a complex NMR spectrum in which the vinylic proton at C2 is coupled with both the C1 vinylic proton (J = 16 Hz) and the C3 methylene protons (J = 8 Hz). Draw a tree diagram for the C2 proton signal, and account for the fact that a five-line multiplet is observed.arrow_forwardExplanation/Answer cannot be hand-drawn! Must be typed or illustrated digitallyarrow_forwardIdentify all the peaks in the H-NMR and C-NMR spectrum. Record the chemical shift, the splitting, and the number of hydrogens for each peak in the NMR table. Please fill in the data similar to the example table down below.arrow_forward
- 6) a)Assign the peaks in the 'H NMR shown below to the correct protons in molecule shown below. The portion of the NMR between 8-6.5 ppm has been magnified for easier viewing. (Hint: Draw resonance structures to distinguish the benzene and alkene Hs. 1H 2H 1H الس 2H CHH BH. CH3 A CH3 D H3C N H CH3 H 6H 6H PPM b) Draw a resonance that shows how you can differentiate the alkene hydrogens in the NMR. It is ok to have more than one positive and negative charge for the resonance structures with a NO2 group.) H H3C-N CH3 CH3 CH3arrow_forwardBelow are the ¹H NMR spectrum of triphenylmethanol, benzophenone, and bromobenzene. Identify the compound corresponding to each ¹H NMR spectrum and draw the structure next to the ¹H NMR spectrum. Assign ALL peaks in each of the three ¹H NMR spectra. Hint: Conjugated systems (benzophenone) including an electronegative atom will cause a more downfield shift of ring protons in ¹H NMR compared with non-conjugated systems (bromobenzene). 8 8 8 7 7 7 6 6 6 5 5 5 4 PPM 4 PPM 4 PPM 3 3 3 2 2 2 1 1 1 0 0 0arrow_forwardIn the attached H1-NMR, draw and identify each set of equivalent hydrogens in the structure and list peak they belong to in the NMR spectrum.arrow_forward
- 1. How many different sets of equivalent aromatic (benzene ring) carbons can be seen in the 13C NMR spectrum of your unknown monosubstituted benzene starting material? A. Are there any peaks for non-aromatic carbons in the 13C NMR spectrum of your unknown monosubstituted benzene starting material (yes or no)? B. If yes for part A, please list the corresponding ppm value(s) for any non-aromatic carbon(s)?arrow_forwardThe product molecule of the experiment would produce 12 signals had we obtained an H¹-NMR spectrum. Label each proton group clearly on the molecule structure and list them with the correct multiplicity and the appropriate chemical shift range from the chemical shift chart. H3Carrow_forward5. Dibromomethane contains 2 equivalent protons, so just one signal on the H NMR spectrum for dibramomethane is expected to be seen. determine the expected chemical shift for the dibromomethane signal.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning


Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole