
Principles of Modern Chemistry
8th Edition
ISBN: 9781305079113
Author: David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
help please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all working!
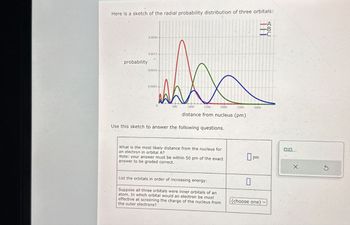
Transcribed Image Text:Here is a sketch of the radial probability distribution of three orbitals:
0.0020
probability
0.0015
0.0010-
0.0005
500
1000
1500
2000
2500
3000
distance from nucleus (pm)
Use this sketch to answer the following questions.
What is the most likely distance from the nucleus for
an electron in orbital A?
Note: your answer must be within 50 pm of the exact
answer to be graded correct.
List the orbitals in order of increasing energy:
Suppose all three orbitals were inner orbitals of an
atom. In which orbital would an electron be most
effective at screening the charge of the nucleus from
the outer electrons?
☐
pm
(choose one)
-B
0,0....
G
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- The energies of macroscopic objects, as well as those of microscopic objects, are quantized, but the effects of the quantization are not seen because the difference in energy between adjacent states is so small. Apply Bohr’s quantization of angular momentum to the revolution of Earth (mass6.01024kg) , which moves with a speed of 3.0104ms1 in a circular orbit (radius1.51011m) about the sun. The sun can be treated as fixed. Calculate the value of the quantum number n for the present state of the Earthsun system. What would be the effect of an increase in n by 1?arrow_forward• identify an orbital (as 1s, 3p, etc.) from its quantum numbers, or vice versa.arrow_forwardUse the mathematical expression for the 2pz wave function of a one-electron atom (see Table 5.2) to show that the probability of finding an electron in that orbital anywhere in the x-y plane is 0. What are the nodal planes for a dxz orbital and for a dx2y2 orbital?arrow_forward
- The wave function of an electron in the lowest (that is, ground) state of the hydrogen atom is (r)=( 1 a 0 3 )1/2exp(r a 0 )ao=0.5291010m (a) What is the probability of finding the electron inside a sphere of volume 1.0pm2 , centered at the nucleus (1pm=1012m) ? (b) What is the probability of finding the electron in a volume of 1.0pm2 at a distance of 52.9 pm from the nucleus, in a fixed but arbitrary direction? (c) What is the probability of finding the electron in a spherical shell of 1.0 pm in thickness, at a distance of 52.9 pm from the nucleus?arrow_forwardConstruct an energy level diagram showing all orbitals for the hydrogen atom up to n=5, labeling each orbital with its appropriate quantum numbers. How many different orbitals are in each shell?arrow_forward6.32 What are the mathematical origins of quantum numbers?arrow_forward
- ow does probability ?t into the description of the atom?arrow_forwardDefend and criticize Bohrs model. Why was it reasonable that such a model was proposed, and what evidence was there that it "works"? Why do we no longer "believe" in it?arrow_forward6.106 When Bohr devised his model for the atom, was he using deductive or inductive reasoning? Explain your answer.arrow_forward
- How does probability fit into the description of the atom?arrow_forward(a) Use the radial wave function for the 3p orbital of a hydrogen atom (see Table 5.2) to calculate the value of r for which a node exists. (b) Find the values of r for which nodes exist for the 3s wave function of the hydrogen atom.arrow_forwardWhat are the allowed values for each of the four quantum numbers: n, l, ml, and ms?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning


Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning