
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
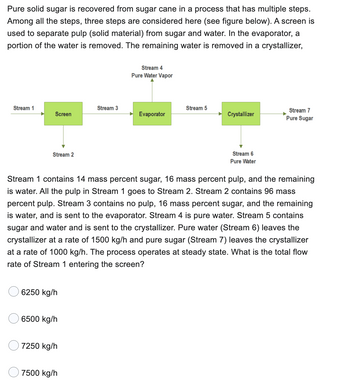
Transcribed Image Text:Pure solid sugar is recovered from sugar cane in a process that has multiple steps.
Among all the steps, three steps are considered here (see figure below). A screen is
used to separate pulp (solid material) from sugar and water. In the evaporator, a
portion of the water is removed. The remaining water is removed in a crystallizer,
Stream 1
Screen
Stream 2
6250 kg/h
6500 kg/h
7250 kg/h
Stream 3
7500 kg/h
Stream 4
Pure Water Vapor
Evaporator
Stream 5
Stream 1 contains 14 mass percent sugar, 16 mass percent pulp, and the remaining
is water. All the pulp in Stream 1 goes to Stream 2. Stream 2 contains 96 mass
percent pulp. Stream 3 contains no pulp, 16 mass percent sugar, and the remaining
is water, and is sent to the evaporator. Stream 4 is pure water. Stream 5 contains
sugar and water and is sent to the crystallizer. Pure water (Stream 6) leaves the
crystallizer at a rate of 1500 kg/h and pure sugar (Stream 7) leaves the crystallizer
at a rate of 1000 kg/h. The process operates at steady state. What is the total flow
rate of Stream 1 entering the screen?
Crystallizer
Stream 6
Pure Water
Stream 7
Pure Sugar
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Similar questions
- One way to make coal “cleaner” is to gasify the coal into “city gas”. In a proposed molten-iron coal gasification process pulverized coal of up to 3 mm size is blown into a molten iron bath and oxygen and steam are blown in from the bottom of the vessel. Materials such as lime for settling the slag, or steam for batch cooling and hydrogen generation can be injected at the same time. The sulfur in the coal reacts with lime to form calcium sulfide, which dissolves into the slag. The process operates at atmospheric pressure and 1400 to 1500°C. Under these conditions, coal volatiles escape immediately and are cracked. The carbon conversion rate is said to be above 98% and the gas is typically 65 to 70% CO, 25 to 35% hydrogen, and less than 2% carbon dioxide. Sulfur content of the gas is less than 20 ppm. Assume that the product gas is 68% Co, 30% H2, and 2% CO2. Calculate the enthalpy change that occurs on the cooling of 1000 m3 of the gas at 1475 °C and 1 atm to 25°C and 1 atm.arrow_forwardThe vapor leaving the top of a distillation column goes to a condenser in which either total or partial condensation takes place. If a total condenser is used, a portion of the condensate is returned to the top of the column as refcr and the remaining liquid is taken off as the overhead product (or distillate). If a partial condenser is used, the liquid condensate is returned as reflux and the uncondensed vapor is taken off as the overhead product. The overhead product from an n-butane-n-pentane distillation column is 93 mole% butane. The temperature of the cooling fluid limits the condenser temperature to 39 °C or higher. REFLUX DRUM a) Using Raoult's law, estimate the minimum pressure at which the condenser can operate as a partial condenser (i.e., at which it can produce liquid for reflux) b) The production rate of overhead product is 50 kmol/h, and the mole ratio of reflux to overhead product is 2:1. Calculate the molar flow rates and compositions of the reflux stream and the vapor…arrow_forward4. [Scrubber]. A coal-fired combustion system produces flue gas that contains particulate matter. To reduce the amount of particles, the flue gas is treated in a scrubber. The measurement of the mass and size of the particles at the inlet and outlet of the scrubber yielded the size distribution data given in the following table: Particle diameter [um] (size interval: dlower → dupper) 0.10.25 0.25 → 0.5 0.5 → 1.0 1.0→2.0 2.05.0 Formulas for question 4: . Particle mass at scrubber inlet [mg] 623 55.4 12.6 8.9 7.1 5.0 10 7.7 10 →30 6.6 30→ 50 4.3 The mass of particles in each size interval was measured on a basis of 1 m²³. 31.4 33.2 123 a) What is the overall efficiency of the scrubber? [Ans: noverall 96.7 %] b) Calculate the collection efficiency (na,i) of the scrubber and the particle geometric average diameter (dpi) for each size interval. Create a graph showing the variation of na,i with dp,i. (PTO) efficiency: nd,i (%) = - 394 775 1055 c) Calculate the mass-average diameter of the…arrow_forward
- 1.) In the production of soybean oil, dried and flaked soybeans are brought into contact with a solvent (often that solvent is hexane) that extracts the oil and leaves behind residual solids and a small amount of oil.(a) Draw a flowchart of the process. Label the two feed streams (soybeans and solvent) and the leaving streams (residual solvents and extract).(b) The soybeans contain 18.5 wt% oil and the remainder insoluble solids and the hexane is fed at a rate corresponding to 2 kg hexane per kg of soybeans. The residual solids leaving the extraction unit contain 35.0 wt% hexane, as well as all of the “non-oil” solids that entered with the soybeans, and 1.0 wt% oil that entered with the beans. The feed rate of dried, flaked soybeans is 1000 kg/h.Correctly identify the basis you will use to solve this material balance.(c) Determine the degrees of freedom for this problem. Can the problem be solved?(d) Based on the quantities listed in part (b), calculate the mass flow rates of extract…arrow_forward4. A 10% (by mass) calcium carbonate slurry (the rest is water) is fed to a drum filter at a rate of 100 kg/h, and water is sprayed on to it at a rate of 5 kg/h. The filtrate (pure water) is collected at a rate of 93.5 kg/h. (a) Sketch a flow diagram for the operation. (b) Calculate the moisture content of the cake.arrow_forwardYou work in a chemical plant which recovers gold from gold-plated electrical connectors using heated (Tacid = 55oC) nitric acid. Once the acid is “used up” it gets piped through the plant to a separate facility for disposal. During that time, it passes through a cooling chamber to strip the waste heat off the acid for recovery. The acid passes through the chamber in 6m long, 400mm OD, 360mm ID aluminum tubes. The chamber passes room-temperature air over the tubes at 8 m/s. Approximately 0.45 cubic meters of acid is processed per hour. a) What is the Reynold’s number of the nitric acid passing through the tube? Is it turbulent or laminar? b) What is the heat transfer coefficient of the nitric acid to the inside of the aluminum tube? c) What is the heat transfer coefficient of the air over the tube? d) What is the overall heat rate from the nitric acid to the air? e) Based on your answers here, in a gas-liquid heat exchanger, which side of the exchanger are the fins usually on?…arrow_forward
- A student uses a polystyrene calorimeter to determine the enthalpy of reaction of hydrobromic acid with potassium hydroxide. The student mixes 100.0 mL of 0.50 mol/L HBr(aq) at 21.0oC with 100.0 mL of 0.50 mol/L KOH(aq), also at 21.0oC. The highest temperature reached is 24.4oC. Write the thermochemical equation of the reaction.arrow_forwardMagnesium metal, a gray solid, is heated in a crucible in the presence of oxygen. A white powder is collected from the crucible. This is an example of A) a chemical change B) a separation C) a mixture D) a physical changearrow_forwardshow psychrometric chart grapharrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The