
Chemistry for Engineering Students
4th Edition
ISBN: 9781337398909
Author: Lawrence S. Brown, Tom Holme
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please correct answer and don't use hand rating
Transcribed Image Text:pts
estion 3 of 7 >
Macmillan Learning
C
N
Cl
HO
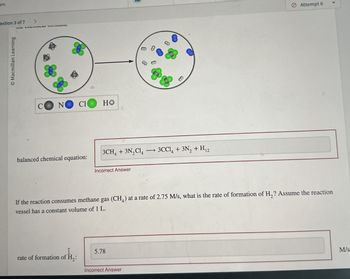
balanced chemical equation:
8
8
8
8
3CH4 + 3N2C14 3CCl4 + 3N2 + H12
Incorrect Answer
Attempt 6
If the reaction consumes methane gas (CH4) at a rate of 2.75 M/s, what is the rate of formation of H₂? Assume the reaction
vessel has a constant volume of 1 L.
I
rate of formation of H₂:
5.78
Incorrect Answer
M/s.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Candle wax is a mixture of hydrocarbons. In the reaction of oxygen with candle w ax in Figure 11.2, the rate of consumption of oxygen decreased with time after the flask was covered, and eventually' the flame went out. From the perspective of the kinetic-molecular theory, describe what is happening in the flask. FIGURE 11.2 When a candle burns in a closed container, the flame will diminish and eventually go out. As the amount of oxygen present decreases, the rate of combustion will also decrease. Eventually, the rate of combustion is no longer sufficient to sustain the flame even though there is still some oxygen present in the vessel.arrow_forwardIodomethane (CH3I) is a commonly used reagent in organic chemistry. When used properly, this reagent allows chemists to introduce methyl groups in many different useful applications. The chemical does pose a risk as a carcinogen, possibly owing to iodomethanes ability to react with portions of the DNA strand (if they were to come in contact). Consider the following hypothetical initial rates data: [DNA]0 ( mol/L) [CH3I]0 ( mol/L) Initial Rate (mol/Ls) 0.100 0.100 3.20 104 0.100 0.200 6.40 104 0.200 0.200 1.28 103 Which of the following could be a possible mechanism to explain the initial rate data? MechanismIDNA+CH3IDNACH3++IMechanismIICH3ICH3++ISlowDNA+CH3+DNACH3+Fastarrow_forwardThe rate of the decomposition of hydrogen peroxide, H2O2, depends on the concentration of iodide ion present. The rate of decomposition was measured at constant temperature and pressure for various concentrations of H2O2and of KI. The data appear below. Determine the order of reaction for each substance, write the rate law, and evaluate the rate constant. Rate [H2OJ [Kll (mL min-’) (mol L ’) (mol L ’) 0.090 0.15 0.033 0.178 0.30 0.033 0.184 0.15 0.066arrow_forward
- 11.41 For a drug to be effective in treating an illness, its levels in the bloodstream must be maintained for a period of time. One way to measure the level of a drug in the body is to measure its rate of appearance in the urine. The rate of excretion of penicillin is first order, with a half-life of about 30 min. If a person receives an injection of 25 mg of penicillin at t = 0, how much penicillin remains in the body after 3 hours?arrow_forwardConsider the following hypothetical reaction: 2AB2(g)A2(g)+2B2(g)A 500.0-mL flask is filled with 0.384 mol of AB2. The appearance of A2 is monitored at timed intervals. Assume that temperature and volume are kept constant. The data obtained are shown in the table below. (a) Make a similar table for the disappearance of AB2. (b) What is the average rate of disappearance of AB2 over the second and third 10-minute intervals? (c) What is the average rate of appearance of A2 between t=30 and t=50?arrow_forwardIn the nuclear industry, chlorine trifluoride is used to prepare uranium hexafluoride, a volatile compound of uranium used in the separation of uranium isotopes. Chlorine trifluoride is prepared by the reaction CI2(g)+3F2(g)2CIF3(g). Write the equation that relates the rate expressions for this reaction in terms of the disappearance of Cl2 and F2 and the formation of CIF3.arrow_forward
- Regular ?ights of supersonic aircraft in the stratosphere ale of concern because such aircraft produce nitric oxide, NO, as a byproduct in the exhaust of their engines. Nitric oxide reacts with ozone, and it has been suggested that this could contribute to depletion of the ozone layer. The reaction NO+O3NO2+O2 is first order with respect to both NO and O3 with a rate constant of 2.20107 L/mol/s. What is the instantaneous rate of disappearance of NO when [NO]=3.3106 M and [O3]=5.9107M?arrow_forwardThe reaction NO(g) + O,(g) — NO,(g) + 0(g) plays a role in the formation of nitrogen dioxide in automobile engines. Suppose that a series of experiments measured the rate of this reaction at 500 K and produced the following data; [NO] (mol L ’) [OJ (mol L 1) Rate = -A[NO]/Af (mol L_1 s-1) 0.002 0.005 8.0 X 10"'7 0.002 0.010 1.6 X 10-'6 0.006 0.005 2.4 X IO-'6 Derive a rate law for the reaction and determine the value of the rate constant.arrow_forwardThe reaction for the Haber process, the industrial production of ammonia, is N2(g)+3H2(g)2NH3(g) Assume that under certain laboratory conditions ammonia is produced at the rate of 6.29 ×10-5 molL-1s-1. At what rate is nitrogen consumed? At what rate is hydrogen consumed?arrow_forward
- Consider the following statements: In general, the rate of a chemical reaction increases a bit at first because it takes a while for the reaction to get warmed up. After that, however, the rate of the reaction decreases because its rate is dependent on the concentrations of the reactants, and these are decreasing. Indicate everything that is correct in these statements, and indicate everything that is incorrect. Correct the incorrect statements and explain.arrow_forwardGo to the PhET Reactions and change to Angled shot to see the difference. (a) What happens when the angle of the collision is changed? (b) Explain how this is relevant to rate of reaction.arrow_forwardRelate the rate of decomposition of NH4NO2 to the rate of formation of N2 for the following reaction: NH4NO2(aq)N2(g)+2H2O(l)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning