Propylene oxide is a chiral molecule. Hydrolysis of propylene oxide gives propylene glycol, another chiral molecule. A. Provide a mechanism for the acid-catalyzed hydrolysis of pure (R)-propylene oxide (treatment with H2SO4/H2O), showing the correct stereochemistry. B. Provide a mechanism for the treatment of pure (R)-propylene oxide with NaOH in water (base-catalyzed hydrolysis), showing the correct stereochemistry. C. Explain why acid-catalyzed hydrolysis of optically active propylene oxide gives a product with a rotation in the opposite direction from the product of the base catalyzed hydrolysis.
Propylene oxide is a chiral molecule. Hydrolysis of propylene oxide gives propylene glycol, another chiral molecule.
A. Provide a mechanism for the acid-catalyzed hydrolysis of pure (R)-propylene oxide (treatment with H2SO4/H2O), showing the correct stereochemistry.
B. Provide a mechanism for the treatment of pure (R)-propylene oxide with NaOH in water (base-catalyzed hydrolysis), showing the correct stereochemistry.
C. Explain why acid-catalyzed hydrolysis of optically active propylene oxide gives a product with a rotation in the opposite direction from the product of the base catalyzed hydrolysis.

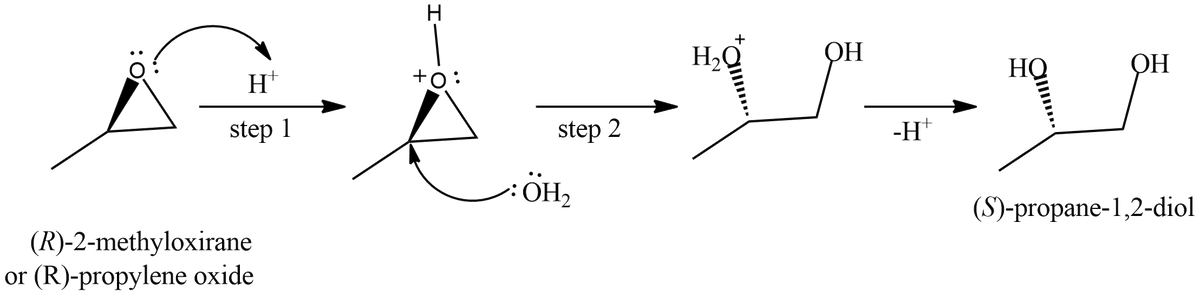
A. The mechanism of acid catalyzed hydrolysis of (R)-propylene oxide (treatment with H2SO4/H2O) involves two steps:
1. In step 1 the (R)-propylene oxide is protonated
2. In step 2 the protonated epoxide is attacked by nucleophile (here, H2O) at most substituted position.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images









