
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN: 9781305079250
Author: Mark S. Cracolice, Ed Peters
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:esc
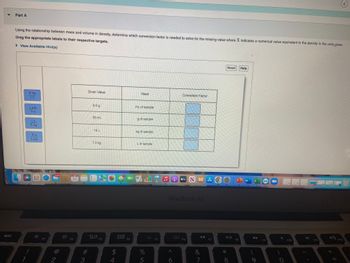
Part A
Using the relationship between mass and volume in density, determine which conversion factor is needed to solve for the missing value where X indicates a numerical value equivalent to the density in the units given.
Drag the appropriate labels to their respective targets.
► View Avallable Hint(s)
X kg
1L
1 mL
Xg
1L
X kg
Xg
1 mL
F1
2
30
F2
Given Value
#
3
5.0 g
55 mL
15 L
7.0 kg
20
F3
S4
$
9.
F4
Need
mL of sample
g of sample
kg of sample
L of sample
%
5
F5
Conversion Factor
6
#tv
MacBook Air
F6
&
✓ lº
AG
A
F7
Reset
(a)
F8
Help
W
F9
F10
!
(<)
F11
112
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Complete the following conversions between SI units. 612g = mg 8.160 m ______ cm 3779 g = ________ g 781 mL = ______ L 4.18 kg = g 27.8 m = ______ km 0.13 mL = ______ L 1738 km ______ m 1.9 Gg = garrow_forward1.65 An industrial engineer is designing a process to manufacture bullets. The mass of each bullet must be within 0.2 5% of 150 grains. What range of bullet masses, in mg, will meet this tolerance? 1 grain = 64.79891 mg.arrow_forwardPerform the following mathematical operations, and express the result to the correct number of significant figures. a. 2.5263.1+0.4700.623+80.7050.4326 b. (6.404 2.91)/(18.7 - 17.1) c. 6.071 105 - 8.2 106 - 0.521 104 d. (3.8 1012 + 4.0 1013)/(4 1012 + 6.3 10l3) e. 9.5+4.1+2.8+3.1754 (Assume that this operation is taking the average of four numbers. Thus 4 in the denominator is exact.) f. 8.9258.9058.925100 (This type of calculation is done many times in calculating a percentage error. Assume that this example is such a calculation; thus 100 can be considered to be an exact number.)arrow_forward
- 1.90 A calibrated flask was filled to the 25.00-mL mark with ethyl alcohol and was found to have a mass of 19.7325 g. In a second experiment, 25.0920 g of metal beads were put into the container and the flask was again filled to the 25.00-mL mark. The total mass of the metal plus the alcohol was 43 .0725 g. Describe how to determine the density of the metal sample.arrow_forwardThe accepted value of the melting point of pureaspirin is 135 C. Trying to verify that value, you obtain 134 C, 136C133 C, and 138 C infour separate trials. Your partner finds 138 C,137 C, 138 C, and 138 C. (a) Calculate the average value and percent error for your data and your partner's data. (b) Which of you is more precise? More accurate?arrow_forwardYou are asked to identify an unknown liquid that is known to be one of the liquids listed below. You pipet a 3.50-mL sample into a beaker. The empty beaker had a mass of 12.20 g. and the beaker plus the liquid weighed 16.08 g. (a) Calculate the density and identify the unknown. (b) If you were able to measure the volume to only two significant figures (that is, 3.5 mL, not 3.50 mL), will the results be sufficiently accurate to identify the unknown? Explainarrow_forward
- Calculate these masses. What is the mass of 6.00 cm3 of mercury, density = 13.5939 g/cm3 What is the mass of 25.0 mL octane, density = 0.702 g/cm3arrow_forwardYou set out to determine the density of lead in the laboratory. Using a top loading balance to determine the mass and the water displacement method (Study Question 41) to determine the volume of a variety of pieces of lead, you calculate the following densities: 11.6 g/cm3, 11.8 g/cm3, 11.5 g/cm3, and 12.0 g/cm3. You consult a reference book and find that the accepted value for the density of lead is 11.3 g/cm3. Calculate your average value, percent error, and standard deviation of your results.arrow_forwardCalculate these masses. What is the mass of 4.00 cm3 of sodium, density = 0.97 g/cm3 What is the mass of 125 mL gaseous chlorine, density = 3.16 g/Larrow_forward
- Vanadium metal is added to steel to impart strength. The density of vanadium is 5.96 g/cm3. Express this in SI units (kg/m3).arrow_forward1-19 Multiply: (a) (2.16 × 105) (3.08 × 1012) (b) (1.6 × 10-8) (7.2 × 108) (c) (5.87 × 1010) (6.6 × 10-27) (d)(5.2 × 10-9)(6.8 × 10-15)arrow_forward1-98 The antifreeze-coolant compound used in cars does not have the same density as water. Would a hydrometer be useful for measuring the amount of antifreeze in the cooling system?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
