
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
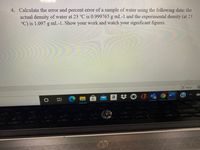
Transcribed Image Text:4. Calculate the error and percent error of a sample of water using the following data: the
actual density of water at 23 °C is 0.999765 g mL-1 and the experimental density (at 23
°C) is 1.097 g mL-1. Show your work and watch your significant figures.
O, Focus
a
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A chemistry student needs 15.0 g of heptane for an experiment. He has available 0.50 kg of a 44.3% w/w solution of heptane in diethyl ether. Calculate the mass of solution the student should use. If there's not enough solution, press the "No solution" button. Round your answer to 3 significant digits. g x10 No solutionarrow_forwardDUE NOW. Please answer it with the correct significant figures. Thank you!arrow_forwardA chemist makes 0.440 L of mercury(I) chloride (Hg,Cl,) working solution by adding distilled water to 0.0800 L of a 0.00000513 M stock solution of mercury(I) chloride in water. Calculate the concentration of the chemist's working solution. Round your answer to 3 significant digits. | M x10arrow_forward
- Express the result of each mathematical operation to the proper number of significant figures and with the correct unit. a) (6.521 g/mL)(0.060 mL) = 1.50 ×10 atoms b) 6.022 x 10 atoms/molearrow_forwardThe standard definition of a quart is equal to 947 mL. Calculate the percent error of a student's measurement if she found the experimental value of the volume of one quart of water to be equal to 938 mL.arrow_forwardGaseous ethane (CH,CH,) will react with gaseous oxygen (O2) to produce gaseous carbon dioxide (CO2) and gaseous water (H,O). Suppose 0.601 g of ethane is mixed with 2.9 g of oxygen. Calculate the maximum mass of water that could be produced by the chemical reaction. Be sure your answer has the correct number of significant digits. g x10 ?arrow_forward
- A student prepares a 0.70 mM aqueous solution of butanoic acid (C,H,CO2H). Calculate the fraction of butanoic acid that is in the dissociated form in his solution. Express your answer as a percentage. You will probably find some useful data in the ALEKS Data resource. Round your answer to 2 significant digits. ① % X 나arrow_forwardHow will each of the following experimental errors influence the results of your calculated densities you reported? State whether the error will tend to make your density results too large, too small or unchanged. Explain why this would occur by identifying which measurements would be affected (high or low) and how this would affect the calculated density. Use the followingIn method 2, the metal cylinder you used had a number of air bubbles on its when immersed in the waterarrow_forwardBefore investigating the scene, the technician must dilute the luminol solution to a concentration of 5.00 x 10-2 M. The diluted solution is then placed in a spray bottle for application on the desired surfaces. How many moles of luminol are present in 2.00 L of the diluted spray? Express your answer with the appropriate units.arrow_forward
- A chemist makes 380.mL of magnesium fluoride MgF2 working solution by adding distilled water to 20.0mL of a 18.8μmolL stock solution of magnesium fluoride in water. Calculate the concentration of the chemist's working solution. Round your answer to 3 significant digits.arrow_forward11 of 11 I Review | Constants Periodic Table Suppose that you want to find the equation for a line that passes through the two points (0,3) and (4, 9). What is the slope of this line? Express your answer numerically. > View Available Hint(s) Πνα ΑΣΦ ? m = Submit rt C Complete previous part(s) 9:41 PM 36%arrow_forwardA student standardized a sodium hydroxide solution with KHP and calculated that the concentration of the sodium hydroxide solution was 0.1001 M. If the solution's concentration was 0.1701 M, what is the relative percent error in the student's result? Anser with two decimal places. ANS Your Answer: Answer I units lyarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY