
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
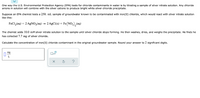
Transcribed Image Text:One way the U.S. Environmental Protection Agency (EPA) tests for chloride contaminants in water is by titrating a sample of silver nitrate solution. Any chloride
anions in solution will combine with the silver cations to produce bright white silver chloride precipitate.
Suppose an EPA chemist tests a 250. mL sample of groundwater known to be contaminated with iron(II) chloride, which would react with silver nitrate solution
like this:
FeCl,(aq) + 2 AgNO3(aq) → 2 AgCl(s) + Fe(NO3),(aq)
The chemist adds 10.0 mM silver nitrate solution to the sample until silver chloride stops forming. He then washes, dries, and weighs the precipitate. He finds he
has collected 7.7 mg of silver chloride.
Calculate the concentration of iron(II) chloride contaminant in the original groundwater sample. Round your answer to 2 significant digits.
mg
L
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- 12.) A 2.00g sample of limestone was dissolved in hydrochloric acid and all the calcium present in the sample was converted to Ca²+, oxalate solution, (NH,),C,O(aqo), was added to the solution to precipitate the calcium ions as calcium oxalate, CaC,O4(s). The precipitate was filtered, dried and weighed to a constant mass of 2.43g. Determine the percentage by mass of calcium in the limestone sample. Excess ammonium (aq)•arrow_forwardA titration is a procedure for determining the concentration of a solution by allowing it to react with another solution of known concentration (called a standard solution). Acid-base reactions and oxidation- reduction reactions are used in titrations. For example, to find the concentration of an HCl solution (an acid), a standard solution of NaOH (a base) is added to a measured volume of HCI from a calibrated tube called a buret. An indicator is also present and it will change color when all the acid has reacted. Using the concentration of the standard solution and the volume dispensed, we can calculate molarity of the HCl solution. A volume of 90.0 mL of aqueous potassium hydroxide (KOH) was titrated against a standard solution of sulfuric acid (H₂SO4). What was the molarity of the KOH solution if 13.2 mL of 1.50 M H₂SO4 was needed? The equation is 2KOH(aq) + H₂SO4 (aq)→K₂SO4 (aq) + 2H₂O(1) Express your answer with the appropriate units. ► View Available Hint(s) molarity = Submit…arrow_forwardTo measure the amount of chlorine in a well-boring fluid, an analytical chemist adds 0.390M silver nitrate (AgNO,) solution to a 45.0 g sample of the fluid and collects the solid silver chloride (AgCl) product. When no more AgCl is produced, she filters, washes and weighs it, and finds that 1.8 g has been produced. The balanced chemical equation for the reaction is: Cl(aq) + AgNO, (aq) What kind of reaction is this? AgCl(s) + NO, (aq) If you said this was a precipitation reaction, enter the chemical formula of the precipitate. If you said this was an acid-base reaction, enter the chemical formula of the reactant that is acting as the base. If you said this was a redox reaction, enter the chemical symbol of the element that is oxidized. Calculate the mass percent of Cl in the sample. Be sure your answer has 2 significant digits. precipitation O acid-base O redox 0 0 0 0% 0° 0 0..9 X 5 Aarrow_forward
- What is the component formed during the hydroboration (BH3, ) oxidation of an alkyne trialkenyl borane osmate carbocation trialkylborane Boranyl cationarrow_forwardSodium hydroxide is extremely soluble in water. At a certain temperature, a saturated solution contains 565 g NaOH(s) per liter of solution. Calculate the molarity of this saturated NaOH(aq) solution.arrow_forward[References] Use the References to access important values if needed for this question. When the following molecular equation is balanced using the smallest possible integer coefficients, the values of these coefficients are: Fe (s) + HCI (aq) →→→→ FeCl₂ (aq) + H₂ (g) 0arrow_forward
- How many grams of Cu(OH)2 will precipitate when excess Ba(OH), solution is added to 61.0 mL of 0.744 M Cu(NO3)2 solution? Cu(NO3)2(aq) + Ba(OH)2(aq) Cu(OH)2(s) + Ba(NO3)2(aq) |garrow_forwardSodium hydrogen carbonate (NaHCO3), also known as sodium bicarbonate or "baking soda", can be used to relieve acid indigestion. Acid indigestion is the burning sensation you get in your stomach when it contains too much hydrochloric acid (HC1), which the stomach secretes to help digest food. Drinking a glass of water containing dissolved NaHCO3 neutralizes excess HCl through this reaction: HCl(aq) + NaHCO3(aq) - NaCl(aq) + H₂O(l) + CO₂(g) The CO₂ gas produced is what makes you burp after drinking the solution. Suppose the fluid in the stomach of a man suffering from indigestion can be considered to be 100. mL of a 0.011 M HCl solution. What mass of NaHCO3 would he need to ingest to neutralize this much HCl ? Round your answer to 2 significant digits. 6.D x10 X Śarrow_forwardOne way the U.S. Environmental Protection Agency (EPA) tests for chloride contaminants in water is by titrating a sample of silver nitrate, solution. Any chloride anions in solution will combine with the silver cations to produce bright white silver chloride precipitate. Suppose an EPA chemist tests a 200. mL sample of groundwater known to be contaminated with cadmium chloride, which would react with silver nitrate solution like this: CdCl2(aq) + 2 AgNO3(aq) → 2 AgCl(s) + Cd (NO3)2(aq) The chemist adds 89.0 mM silver nitrate solution to the sample until silver chloride stops forming. He then washes, dries, and weighs the precipitate. He finds he has collected 5.4 mg of silver chloride. Calculate the concentration of cadmium chloride contaminant in the original groundwater sample. Be sure your answer has the correct number of significant digits. E d OL Earrow_forward
- Sodium hydrogen carbonate (NaHCO3), also known as sodium bicarbonate or "baking soda", can be used to relieve acid indigestion. Acid indigestion is the burning sensation you get in your stomach when it contains too much hydrochloric acid (HCI), which the stomach secretes to help digest food. Drinking a glass of water containing dissolved NaHCO3 neutralizes excess HC1 through this reaction: HCl(aq) + NaHCO3(aq) → NaCl(aq) + H₂O(1) + CO₂(g) The CO2 gas produced is what makes you burp after drinking the solution. Suppose the fluid in the stomach of a man suffering from indigestion can be considered to be 100. mL of a 0.029 M HCl solution. What mass of NaHCO3 would he need to ingest to neutralize this much HCl ? Be sure your answer has the correct number of significant digits. X Śarrow_forwardOne way the U.S. Environmental Protection Agency (EPA) tests for chloride contaminants in water is by titrating a sample of silver nitrate solution. Any chloride anions in solution will combine with the silver cations to produce bright white silver chloride precipitate. Suppose an EPA chemist tests a 250. mL sample of groundwater known to be contaminated with iron(III) chloride, which would react with silver nitrate solution like this: FeCl,(aq) + 3 AgNO3(aq) 3 AgCl(s) + Fe(NO,),(aq) → The chemist adds 17.0 mM silver nitrate solution to the sample until silver chloride stops forming. He then washes, dries, and weighs the precipitate. He finds he has collected 8.2 mg of silver chloride. Calculate the concentration of iron(III) chloride contaminant in the original groundwater sample. Round your answer to 2 significant digits. mg Larrow_forwardSodium hydrogen carbonate (NaHCO3), also known as sodium bicarbonate or "baking soda", can be used to relieve acid indigestion. Acid indigestion is the burning sensation you get in your stomach when it contains too much hydrochloric acid (HCI), which the stomach secretes to help digest food. Drinking a glass of water containing dissolved NaHCO3 neutralizes excess HCl through this reaction: HCl(aq) + NaHCO3(aq) NaCl(aq) + H2O(l) + CO₂(9) The CO2 gas produced is what makes you burp after drinking the solution. Suppose the fluid in the stomach of a woman suffering from indigestion can be considered to be 250. mL of a 0.076 M HCl solution. What mass of NaHCO3 would she need to ingest to neutralize this much HCl ? Round your answer to 2 significant digits. x10 Garrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY