
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
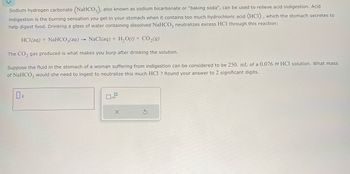
Transcribed Image Text:Sodium hydrogen carbonate (NaHCO3), also known as sodium bicarbonate or "baking soda", can be used to relieve acid indigestion. Acid
indigestion is the burning sensation you get in your stomach when it contains too much hydrochloric acid (HCI), which the stomach secretes to
help digest food. Drinking a glass of water containing dissolved NaHCO3 neutralizes excess HCl through this reaction:
HCl(aq) + NaHCO3(aq) NaCl(aq) + H2O(l) + CO₂(9)
The CO2 gas produced is what makes you burp after drinking the solution.
Suppose the fluid in the stomach of a woman suffering from indigestion can be considered to be 250. mL of a 0.076 M HCl solution. What mass
of NaHCO3 would she need to ingest to neutralize this much HCl ? Round your answer to 2 significant digits.
x10
G
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 5 steps with 8 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- CO A buffer is prepared by mixing hypochlorous acid (HCIO) and sodium hypochlorite (NACIO). If a strong acid, such as HCl, is added to this buffer, which buffer component neutralizes the additional hydrogen ions (H+)? OIDH O Write a balanced chemical equation for the reaction of the selected buffer component and the hydrogen ion (H+). Do not include physical states. chemical equation: CIO + H,O* → H,O + HCIO Incorrect MacBook Air DD. 889 & 7. 6 K Narrow_forwardThe balanced molecular equation for complete neutralization of H 2SO 4 by KOH in aqueous solution is H2SO4 (aq) + 2КОН (аq) — 2H20 (I) + К2S04 (s) B H2SO4 (aq) + 20H- (aq) → 2H2O (I) + SO42- (aq) (C) 2H+ (aq) + 20H- (aq) → 2H2O (I) D H2SO4 (aq) + 2KOH (aq) – 2H2O (I) + K2SO4 (aq) 2H+ (aq) + 2KOH (aq) → 2H2O (I) + 2K+ (aq) E.arrow_forwardThe concentration of SO42– ions in a 45.0 mL sample of seawater is determined by adding a solution of BaCl2 and precipitating the SO42– as BaSO4. After the precipitate is filtered from the solution, it is dried and weighed. If the mass of BaSO4 recovered is 0.315 g, what is the sulfate concentration of the seawater sample? Express your answer in mmol/L.arrow_forward
- using this balanced equation CaCO3(s) + 2 HCl(aq) ----> H2O(l) + CO2(g) + CaCl2(aq) Stomach acid is 0.100 M HCL. An active ingreident found in antacids such as Alka Seltzer is calcium carbonate, CaCO3. Calculate grams of calcium carbonate needed to react with 80 ml of stomach acid.arrow_forwardA) A student completely dissolves 5.16 mol of Ca(OH)2 in water. i) Which equation is the dissociation equation for Ca(OH), in water? (Give the letter choice)| A. Ca(OH) 2 (s) + H 20 ---> CaO(aq) + O 2 (g) + 2 H 2 (g) С. Са(ОН) 2 (s) --> Са 2+ (aq) + 20 2- (aq) + 2H * (aq) В. Cа(ОН) 2 (s) ---> Са 2" (аq) + 2 ОН (аq) D. Ca(ОН) 2 (s) --> CаН 2 (aq) + O 2 (g) ii) How many total moles of ions are released from the 5.16 mol of Ca(OH)2 ? mol of ions B) A sample of FeCl3 is completely dissolved in water. i) Which is the dissociation equation for FeCl3 in water? (Give the letter choice) A. FeCl 3 (s) + H 20 ---> FeCl 2 (aq) + HCI(aq) + OH (aq) C. FeCl (s) ---> Fe 3+ (aд) + Cl 2 (aq) + Cl (aq) 3 В. FeCl 3 (s) + Н20 ---> FEHCI (aq) + ОН " (aq) D. FeCl 3 (s) ---> Fe 3+ (ад) + 3 CI (аq) ii) How many total moles of ions are released if 5.71 x1019 formula units of FeCl3 were completely dissolved? (NOTE: Write answer in sci. notation using "E" or "e" to replace "x 10" & do NOT leave any space between…arrow_forwardA 6.404 gram sample of sodium hydroxide (NaOH) is mixed with 22.50 mL of a 0.642 M HCl solution. Determine if excess acid or base is present after being mixed. Determine how many moles of excess acid or base is present. Determine the molarity of excess acid or base. Finally determine the pH of the solution. NaOH(s) + HCl(aq) → H2O(L) + NaCl(aq)arrow_forward
- The end point in a titration of 50.00 mL of aqueous NaOH was reached by the addition of 35.23 mL of 0.250 M aqueous HCI titrant. Calculate the molarity of the NaOH solution. The titration reaction equation is: NaOH(aq) + HCI (aq) —> NaCl(aq) + H2O(l)arrow_forwardComplete the equation for the dissociation of Fe(ClO4)3(aq). Omit water from the equation because it is understood to be present. Fe(ClO4)3(aq) -->arrow_forwardA technician would like to standardize an unknown Ca(OH)2 solution. It takes 18.53 mL of a 0.174 M solution of HCI to titrate 25.00 mL the unknown Ca(OH)2 solution. Calculate the molarity of the Ca(OH)2 solution. Tip: Don't forget to consider the mole ratio between HCl and Ca(OH)2. 2 HCI (aq) + Ca(OH), (aq) CaCl2 (aq) + 2 H2O (1)arrow_forward
- A scientist is investigating the solubility of two polyatomic ions, oxalate (C2O2−4C2O42−) and arsenate (AsO3−4AsO43−), which are not listed in the table of solubility guidelines. The scientist starts with four solutions made of water-soluble salts. Solution A contains sodium arsenate. Solution B contains ammonium oxalate. Soution C contains silver chlorate. Solution D contains aluminum bromide. Solution Solute Color of Solution A Na3AsO4Na3AsO4 colorless B (NH4)2C2O4(NH4)2C2O4 colorless C AgClO3AgClO3 colorless D AlBr3AlBr3 yellow The results of mixing each solution in pairs are shown in the table. Experiment Solutions Mixed Result 1 A + B no precipitate, colorless solution 2 A + C brown precipitate 3 A + D white precipitate 4 B + C white precipitate 5 B + D white precipitate 6 C + D yellow precipitate Identify the formula for each precipitate that forms. precipitate from A+C: precipitate from A+D: precipitate…arrow_forwardConsider the reaction: HCI(aq) + NH, (ag) – NH,Cl(aq) Where 20.00 mL of 0.100 M NH, (aq) is added to 10.00 mL of 0.200 M HCl(aq). Order the solution components (excluding water) from highest to lowest concentration. O [OH¯] > [NH3] > [Na*] > [Cl¯] > [H,O*] O (CI ] = [NH†] > [NH,] = [H,O*]> [OH¯] O [H,0*)> [Cl¯] > [NH,] > [NH†]> [OH) O [NH]= [OH"] > [H,O*] = [CI¯] > [NH,] O [H,0*] = [CI¯] > [NH,] > [NH ] = [OH] O [NH,CI] = [NH,] = [HCl] O [CI] > [NH†]> [H,O+] > [NH,] > [OH ] O INH,CI] > [NH3] > [HCl] O None of thesearrow_forwardYou have a 1.153 g sample of an unknown solid acid, HA, dissolved in enough water to make 20.00 mL of solution. HA reacts with KOH(aq) according to the following balanced chemical equation:HA(aq)+KOH(aq) --------->KA(aq)+H2O(l) If 14.90 mL of 0.585 M KOH is required to titrate the unknown acid to the equivalence point, what is the concentration of the unknown acid? ______ M What is the molar mass of HA? _____ g/molarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY