
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
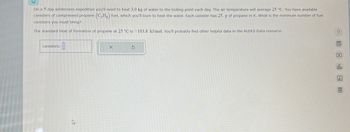
Transcribed Image Text:On a 5 day wilderness expedition you'll need to heat 3.0 kg of water to the boiling point each day. The air temperature will average 25 °C. You have available
canisters of compressed propane (C3H8) fuel, which you'll burn to heat the water. Each canister has 25. g of propane in it. What is the minimum number of fuel
canisters you must bring?
The standard heat of formation of propane at 25 °C is -103.8 kJ/mol. You'll probably find other helpful data in the ALEKS Data resource.
canisters:
13
X
G
000
Ar
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- On a 10 day wilderness expedition you'll need to heat 5.0 kg of water to the boiling point each day. The air temperature will average 25 °C. You have available canisters of compressed butane (C,H10) fuel, which you'll burn to heat the water. Each canister has 100. g of butane in it. What is the minimum number of fuel canisters you must bring? The standard heat of formation of butane at 25 °C is – 125.7 kJ/mol. You'll probably find other helpful data in the ALEKS Data resource. canisters:arrow_forwardOn a 7 day wilderness expedition you'll need to heat 2.0 kg of water to the boiling point each day. The air temperature will average 25 °C. You have available canisters of compressed propane (C,H) fuel, which you'll burn to heat the water. Each canister has 25. g of propane in it. What is the minimum number of fuel canisters you must bring? The standard heat of formation of propane at 25 °C is – 103.85 kJ/mol. You'll probably find other helpful data in the ALEKS Data resource. canisters:arrow_forwardTHe G.R.A.S.P. methodarrow_forward
- In a coffee cup calorimeter, 40.0 mL of 0.33 M nitric acid (HNO3) and 40.0 mL of 0.33 M potassium hydroxide (KOH) are mixed to observe the heat released during the neutralization reaction. Based on the data in the table, what is the enthalpy of the neutralization reaction between HNO3 and KOH? Initial temperature in the calorimeter (°C) Final temperature in the calorimeter (°C) Final mass of the neutralized solution (g) Calorimeter constant (J/C)) 21.3 23.5 79.74 4.57arrow_forwardA bomb calorimeter, or a constant volume calorimeter, is a device often used to determine the heat of combustion of fuels and the energy content of foods. In an experiment, a 1.4710 g sample of maleic acid (C4H4O4) is burned completely in a bomb calorimeter. The calorimeter is surrounded by 1159 g of water. During the combustion the temperature increases from 22.46 to 25.59 °C. The heat capacity of the calorimeter, not including the surrounding water, was determined in a previous experiment to be 852.2 J/°C. Assuming that no energy is lost to the surroundings, calculate the molar heat of combustion of maleic acid based on these data. Assuming that no energy is lost to the surroundings, what is the molar heat of combustion of maleic acid, based on these data? (in kJ/mol). C4H4O4(s) + 3O2(g) → 2 H2O(l) + 4 CO2(g) + Energyarrow_forwardWhen a 4.31 g sample of liquid octane (C8H18) is burned in a bomb calorimeter, the temperature of the calorimeter rises by 27.3 °C. The heat capacity of the calorimeter, measured in a separate experiment, is 6.2 kJ/•C. The calorimeter also contains 3.00 kg of water, specific heat capacity of 4.18 J/g°C. Determine the heat of combustion of octane in units of kJ/mol octane. Enter your answer numerically and in terms of kJ/mol.arrow_forward
- A 3.000 g sample of food was then burned in a calorimeter to determine its Calories per gram content. 50mL of water were used to determine ΔT of the reaction. Initial temperature of the water at the start of the combustion reaction was 20oC, final temperature was 99oC. Calorimeter constant for this calorimeter is 10.0J/oC. How much heat has been absorbed by the water sample?arrow_forward1. Magnesium solid reacts with aqueous hydrochloric acid to form the Mg2+ ion in solution. In an experiment, 60.0 mL of aqueous HCl was mixed with 0.1297 g of magnesium solid in a double Styrofoam cup calorimeter. The reaction caused the temperature of the substances in the calorimeter to rise 10.02°C. Assume the density and specific heat of the HCl solution is that of water, 1.00 g/mL and 4.184 J/g °C, respectively. The specific heat of magnesium is 1.02 J/g °C. a. Write the balanced chemical equation for this reaction. b. Calculate the heat of this reaction, AHrxn, in kJ. c. Calculate the heat of this reaction per mole of Mg2+ formed, AHxn/mole Mg²+. d. The literature value of this reaction is -466.85 kJ/mole. Calculate the percent deviation of the experimental value from the literature value.arrow_forwardOn a 14 day wilderness expedition you'll need to heat 3.0 kg of water to the boiling point each day. The air temperature will average 25 °C. You have available canisters of compressed propane (C₂Hg) fuel, which you'll burn to heat the water. Each canister has 50. g of propane in it. What is the minimum number of fuel canisters you must bring? The standard heat of formation of propane at 25 °C is -103.8 kJ/mol. You'll probably find other helpful data in the ALEKS Data resource. canisters: 0 X Śarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY