
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
6. Determine whether each compound is chiral or achiral. select the single best answer for each part.
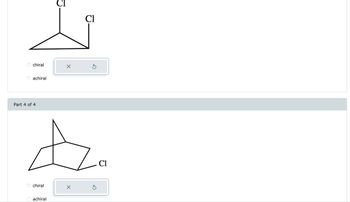
Transcribed Image Text:**Image Description for Educational Website:**
The image displays molecular structures used for determining chirality.
**Top Structure:**
- **Description:** A three-dimensional molecular structure featuring a triangle. Two chlorine atoms (Cl) are attached at different positions.
- **Chirality Options:**
- o chiral
- o achiral
- An incorrect answer indicator (X) is displayed.
**Bottom Structure (Part 4 of 4):**
- **Description:** A bicyclic structure with one chlorine atom (Cl) attached. The molecule appears to have a bridged structure.
- **Chirality Options:**
- o chiral
- o achiral
- An incorrect answer indicator (X) is displayed.
These structures are presented for analysis to determine whether they are chiral or achiral. Chirality in molecules is a key concept in stereochemistry, and involves the presence of a non-superimposable mirror image.
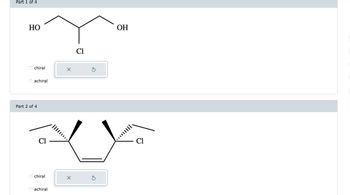
Transcribed Image Text:### Part 1 of 4
#### Molecular Structure:
- A chemical compound is shown with atoms arranged in a specific configuration.
- The central carbon atom is bonded to a hydroxyl group (OH) on the left, a chlorine atom (Cl) below, another carbon atom on the right, and an additional carbon atom above.
- The second carbon atom to the right is bonded to another hydroxyl group (OH).
#### Chirality Options:
- The question asks whether the molecule is chiral or achiral.
- Selection buttons provide two options:
- O chiral
- O achiral
### Part 2 of 4
#### Molecular Structure:
- This diagram displays a cyclopentane ring with two chlorine atoms as substituents.
- Chlorine atoms are positioned at the second and fourth carbon atoms, indicating stereochemistry with wedge and dash bonds:
- The left chlorine is shown with a wedge bond (projecting out).
- The right chlorine is shown with a dashed bond (projecting behind).
#### Chirality Options:
- Similar to Part 1, this part asks for the chirality of the molecule.
- Selection buttons are provided with options:
- O chiral
- O achiral
This educational content is meant to guide learners through identifying chiral centers in molecular structures and distinguish between chiral and achiral compounds based on their stereochemistry.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- a. Use bond energies (see Table 3-3) to estimate H for the reaction of two molecules of glycine to form a peptide linkage. b. Would you predict S to favor the formation of peptide linkages between two molecules of glycine? c. Would you predict the formation of proteins to be a spontaneous process?arrow_forwardIf a drop of oleic acid is added to a dish of water, the oleic acid will spread out and form a layer that is one molecule thick on top of the water. (a) Draw the Lewis structure for oleic acid. (b) Label the region of the oleic acid molecule that is polar and the region that is nonpolar. (c) In the layer of oleic acid that forms, which part of the oleic acid molecule points down into the water and which part points out of the water?arrow_forwardWhat is the meaning of the term tertiary (3) when it is used to classify alcohols? Draw a structural formula for the one tertiary (3) alcohol with the molecular formula C4H10O.arrow_forward
- For example, alanine is a chiral amino acid that has two enantiomers: (+)-alanine and (-)-alanine. These two are optical isomers. NH2 NH2 4. One of the most important properties of chiral molecules in solution is their effect on plane- polarized light, this effect is called optical activity. -C H 2 COOH HOOC CH When an enantiomer rotates a plane-polarized light in the positive direction or clockwise, it is dextrorotary (+ or d), while for a negative direction or counterclockwise, it is levorotary (- or l) (+)-Alanine (-)-Alanine Alanine is a chiral amino acid that has two enantiomers: (+)-alanine and ()-alanine. These two are optical isomers.arrow_forwardFor some questions you will need to use the special periodic table attached in the below images! Treat Je, Qu, Ap, and Bg as NONMETALS! Identify each of the following structures as chiral or achiral AND circle the chiral carbon atoms in each structure that is chiral. (Look at attached images) Please Show your work so I can understand going forward.arrow_forwardPlease help me pleasearrow_forward
- Consider vitamin C, shown below. It contains [1,2,3,or4] chiral carbon atoms and the carbon atom indicates by an arrow has a [linear, trifocals pyramidal, trifocals planar, or tetrahedral] shape.arrow_forward19 The alkane CH3CH2CH(CH3)CH2CH(CH3)2 has how many 1°, 2° and 3° carbon atoms? * O 10: 3, 20: 2, 30 2 O 1°: 5, 20: 2, 30: 1 O 19: 3, 2°: 2, 3°: 2 O 19: 4, 2°: 2, 30: 2arrow_forwardOrganic Chemistry - Select the correct organic structure for the systematic names below. (Can you help me name them all? Technically i only need specific ones but I'd like to learn how to do these all properly)arrow_forward
- The compound shown below is a synthetic estrogen. It is marketed as an oral contraceptive under the name Enovid. In addition to an alkane (cycloalkane), this molecule also contains the following functional groups:arrow_forward1. Predict the structure of ethanol once it oxidizes in the human body. Draw the condensed structure. 2. A student found a bottle in the lab filled with a colorless solution. The label on the bottle was fading. The students were able to correctly identify, but knewit was aa phenol from the readable part of the label. The student decided to run a ferric chloride test. The test resulted in a greed color. What is the identity of the liquid? 3. Predict below alcohols their skeletal structure; if soluble in water; does it contain a phenol group, is it primary, secondary, or tertiary alcohol; can it be oxidized by chromate; if yes what is the oxidation product and draw the skeletal structure; will the alcohol form a colorful ccomplex with Fe3+? Thymol ( found in oil of thyme, antiseptic properties) Menthol ( found in mint, gives a cooling sensation) Cinnamyl aalcohol (used in perfumes ans deodorants) Cety alcohol (ingredient in shampoo, creams, and lotions)arrow_forward(Picture attached) Functional Groups: 1. Benzene 2. Halogen 3. Carboxyl 4. Hydroxyl Identify the functional groups that the 2 molecules contain. Note: each functional group can be used more than once. Put in numerical order with no space. Sucarlose = Ibuprofen =arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning