
Human Anatomy & Physiology (11th Edition)
11th Edition
ISBN: 9780134580999
Author: Elaine N. Marieb, Katja N. Hoehn
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Question
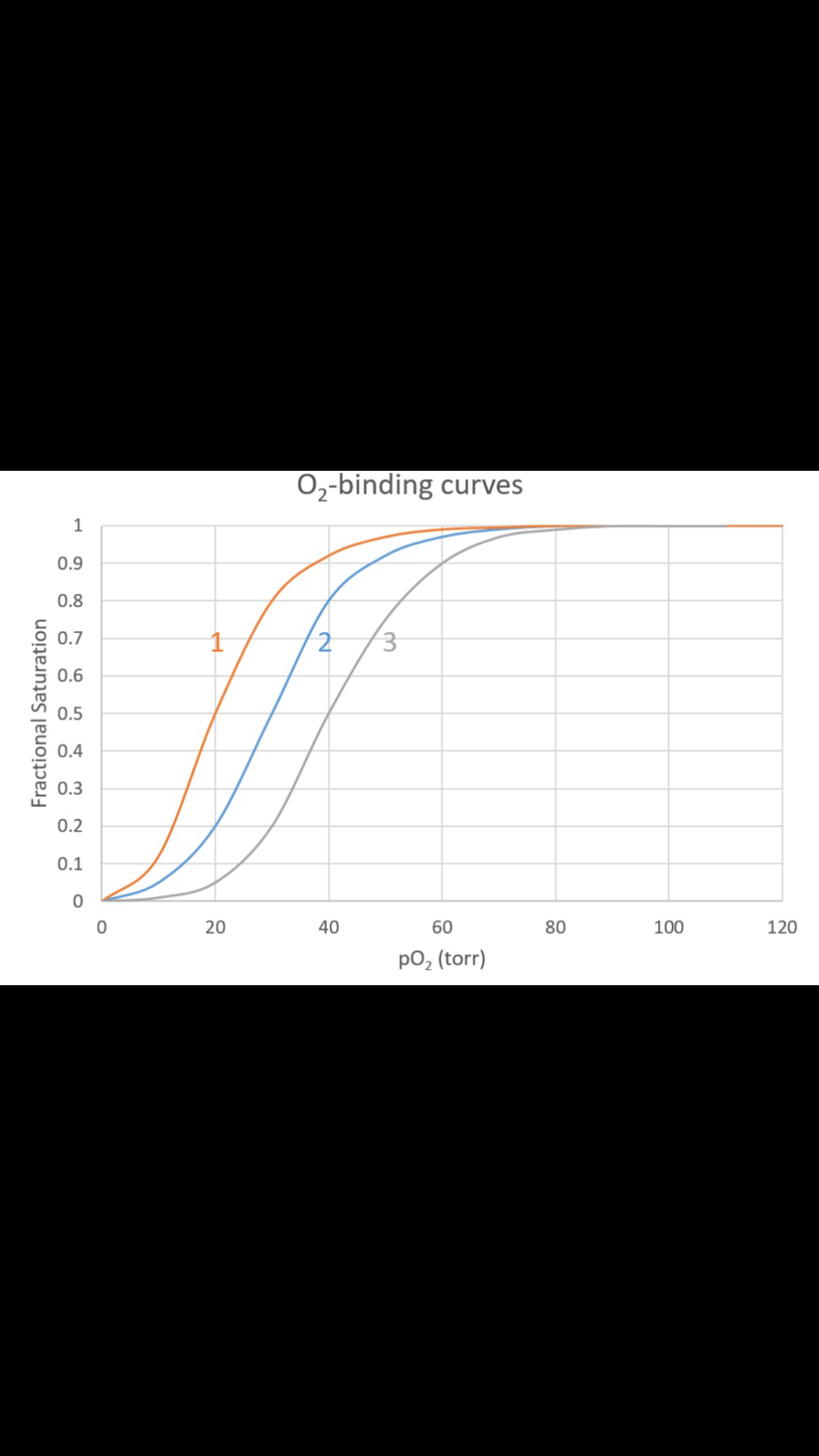
Transcribed Image Text:O2-binding curves
1
0.9
0.8
2
0.7
3
0.6
0.5
0.4
0.3
0.2
0.1
100
120
20
40
60
80
po2 (torr)
Fractional Saturation
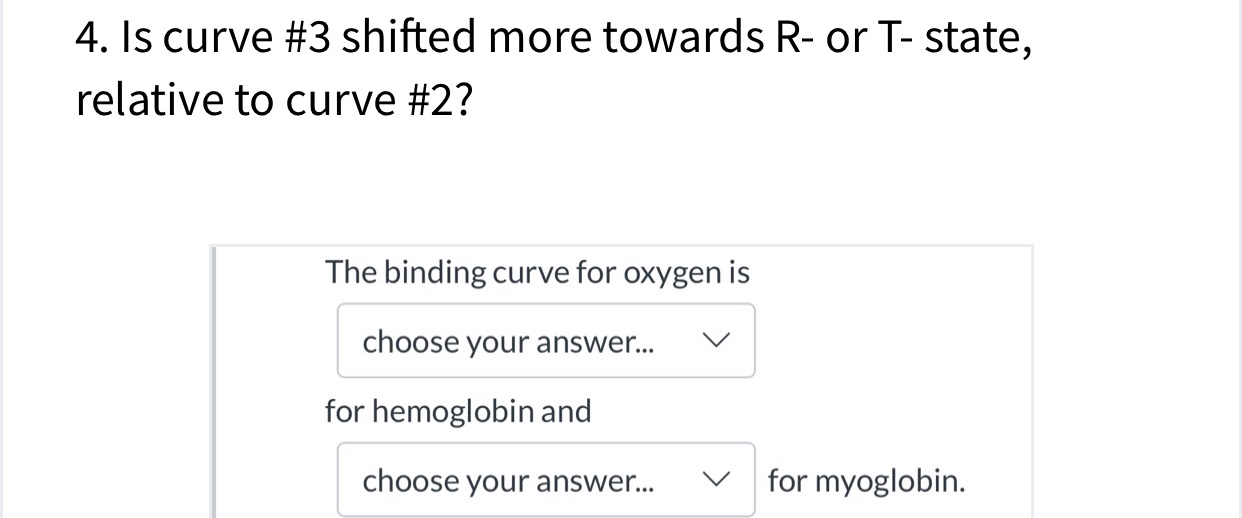
Transcribed Image Text:4. Is curve #3 shifted more towards R- or T- state,
relative to curve #2?
The binding curve for oxygen is
choose your answer...
V
for hemoglobin and
for myoglobin
choose your answer...
V
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Similar questions
- 2. The diagram to the right shows the change in the structure of the C-terminal portion of each of the ẞ-subu- nits of human hemoglobin (HbA) in the oxyHb to deox- yHb or R-to-T transition. The hydrogen bonding interac- tion of the C-terminal ẞHis 146 residue with the side chain of Asp94, highlighted by the red ellipse, has been shown to be responsible for a major portion of the proton uptake associated with the Bohr effect. Treatment of HbA with the enzyme carboxypeptidase A (CPA) results in loss of the C-terminal ẞHis 146 and ẞTyr145 residues of the ẞ- subunits. (a) ( ) Draw a Hill plot [log(Y/[1-Y]) vs. log(pO2), Y = fraction of heme groups occupied by O2] to compare the values of the Hill coefficient nн and the O2-binding affinity at pH 7.4 of normal HbA before and after treat- ment of with CPA. (b) (' ) How will the plot for CPA-digested HbA change at pH 7.2? (c) 1 Hi5146 HN- ✓ Low PK B-chain. CH2 CH-NH-CO-CH-NH- CH₂ Tyr145 он HbO2 or R state Туг145 CH-CH2- OH co NH CH CH₂ His…arrow_forwarddrghhjhrarrow_forward1. All oxygen inhaled by animals and transported around their bodies is destined for specific locations in the body. Once oxygen reaches protein complex IV of the electron transport chain (ETC) it begins its vital task of pulling electrons away from protein complex IV. In short, a set of chain reactions are triggered at protein complex IV. From here 2 electrons are taken from complex III, which then takes two electrons from complex II, or l. a) Use a diagram and your own words to describe how a proton-motive force created by the ETC generates ATP. Make sure you label your diagram correctly and include; inner mitochondrial membrane, hydrogen ions, ADP, P, intermembrane space, ATP synthase, mitochondrial matrix, and ATP.arrow_forward
- You've discovered a new organism that contains a mutant form of Hemoglobin (red line on right) and are testing its properties in a lab setting at a neutral pH. You've generated the binding data below comparing it to the wildtype (non-mutant; blue line on left) form. Oxygen binding of a new hemoglobin mutant 1.00 - 0.75- Hemoglobin 0.50 - Wildtype Mutant 0.25- 0.00 - 25 50 75 100 pO2 (torr) 1. How does this mutation seem to impact cooperativity? Hint. Look at the slope of the line. [ Select ] 2. Which variant, the mutant or wildtype, has a higher affinity for oxygen? [ Select ] 3. Which variant is a more effective delivery system to the the muscles (~20 torr)? Select ] Fraction of Hb bounw with Oxygenarrow_forward6arrow_forwardI now know the structure difference between myoglobin and hemoglobin , however which exctaly structure difference make myglobin become a oxygen storage ? and not a oxygen transport?? Be specific! details describe how the structure connect to their role. also which exctaly structure difference make hemoglobin become better oxygen transport?? I need the specific structure that contribute to their unique role. I KNOW THE STRUCTURE DIFFERENCE , but don't understand which specific part make myoglobin only bind to oxygen, and not release oxygenarrow_forward
- A mutation of Lysine 82 (in the DPG-binding pocket) to Arginine in beta sub-unit will most likely result in Not enough information Higher than normal binding affinity to oxygen Lower than normal binding affinity to oxygen Normal binding to oxygenarrow_forwardCould you help me with this?arrow_forwardChemistry Red cells have a volume of ~86 fL. If the concentration of hemoglobin in red cells is ~330 g/L and its Mr is 64,500 b) In the generation of a red cell, the synthesis of all the hemoglobin molecules of the cell takes about 40 h. How many hemoglobin molecules are synthesized in one second per red cell? c) How many subunits (monomers) of hemoglobin are produced in one minute?arrow_forward
- 5. Patients suffering from Familial Hypercholesterolemia (FH) can carry a mutation in one of several genes. Two of these genes are ApoB and PCSK9. a. Diagram the normal cholesterol sensing pathway for a healthy person. This should be focused on the molecules that handle cholesterol and that regulate cholesterol production, NOT on patient symptoms. b. On your diagram indicate where a mutation in Apoß disrupts cholesterol sensing. Then write a paragraph or two explaining how cholesterol sensing is specifically disrupted in patients with an ApoB mutation. c. On your diagram indicate where a mutation in PCSK9 disrupts cholesterol sensing. Then write a paragraph or two explaining how cholesterol sensing is specifically disrupted in patients with PCSK9 mutation.arrow_forward5. A 70 kg adult could meet her/his entire energy needs for a day by eating 3 moles of glucose (540 g; molecular weight of glucose is 180 g/mol). Each molecule of glucose generates approximately 36 molecules of ATP when oxidized to CO,. The concentration of ATP in cells is about 5mM, and a 70 kg adult has about 25 liters of intracellular fluid. A. Given that the ATP concentration remains constant in cells, calculate how many times per day, on average, each ATP molecule in the body is hydrolyzed and resynthesized. Show calculations.arrow_forward4 8. Rotenone is a chemical that naturally occurs in plants. It is a broad-spectrum poison, meaning it is toxic to many types of organisms, including fish, insects, and other pests. Rotenone inhibits the transfer of electrons from one electron transport chain complex to another (complex I to ubiquinone). a. Would you expect rotenone poisoning to affect the following molecules (yes or no)? If so, would you expect them in increase or decrease? Molecule Acetyl-CoA ATP NADH Will levels change? (Yes or no) Yes No yes If yes, will the levels increase or decrease?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON
Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education,
Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education, Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company
Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co.
Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co. Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education
Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education

Human Anatomy & Physiology (11th Edition)
Biology
ISBN:9780134580999
Author:Elaine N. Marieb, Katja N. Hoehn
Publisher:PEARSON

Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax

Anatomy & Physiology
Biology
ISBN:9781259398629
Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa Stouter
Publisher:Mcgraw Hill Education,

Molecular Biology of the Cell (Sixth Edition)
Biology
ISBN:9780815344322
Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter
Publisher:W. W. Norton & Company

Laboratory Manual For Human Anatomy & Physiology
Biology
ISBN:9781260159363
Author:Martin, Terry R., Prentice-craver, Cynthia
Publisher:McGraw-Hill Publishing Co.

Inquiry Into Life (16th Edition)
Biology
ISBN:9781260231700
Author:Sylvia S. Mader, Michael Windelspecht
Publisher:McGraw Hill Education