
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
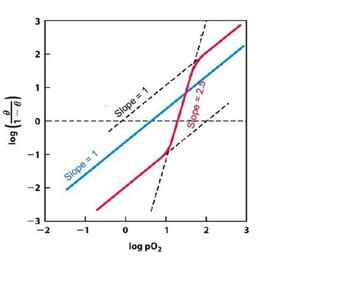
Transcribed Image Text:log (10)
-1
-2
-3
-
2
3
Slope = 1
Slope = 1
-----
-2
0
log po₂
Slope = 2.5
1
2
3

Transcribed Image Text:9.Shown below are the Hill plots of myoglobin (cyan) and an unknown 02 binding protein (magenta).
(a) Does this unknown protein bind to 02 cooperatively? If so, is it a positive or negative
cooperativity?
(b) Does binding of the 1st 02 increase or decrease the affinity for the 2nd O2?
(c)What is the minimal number of 02 binding sites that this unknown protein has? Why?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Which region on the following Ramachandran Plot corresponds to the allowable region(s) for Pro? 120 -120 1,2,4 04 1, 3, 4 60 2,3 120 3 All of the answers are correctarrow_forwardThe order is for Streptomycin 1gm IM. You have a 5gm vial of Streptomycin . The label states to add 9ml of sterile water to yield 400mq / m * l How many ml will you give?arrow_forwardWhich number is identifying the phase which occurs between -55mV and +30mV? A 01 02 O 3 4 O 5arrow_forward
- please dont copy ur answer from chegg since the answers there on this quiestion are not correct/reliablearrow_forwardIdentify each of the following structuresarrow_forwardQuestion 1 Total claims denied: $10,000 Total claims submitted: $100,000 Time period: 3 months What's the claims denial rate?arrow_forward
- The common standard for the maximum time lapse between dinner and breakfast is hours. --- 14 9. 12 10arrow_forwardAssuming the line is not perfect, what are some sources of error? If you state ‘human error’ be specific as to what the human did to cause error.arrow_forwardI asked earlier a question, and I got this answer. Now, my question is: how do you get the 2.5cm= 100um? I have taken a picture of the paramecium in question, with the scale bar (100um), and a ruler showing it’s measurement is around 2.5cm. Is that how we got the 2.5?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON