
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
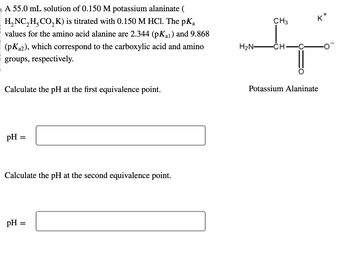
Transcribed Image Text:o A 55.0 mL solution of 0.150 M potassium alaninate (
H₂NC₂H₂CO₂K) is titrated with 0.150 M HCl. The pKa
values for the amino acid alanine are 2.344 (pKal) and 9.868
(pK₂2), which correspond to the carboxylic acid and amino
groups, respectively.
Calculate the pH at the first equivalence point.
pH =
Calculate the pH at the second equivalence point.
pH =
H₂N-
CH3
-CH-
K
Potassium Alaninate
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 15.0 mL of KOH 100.0 mL of KOH 35.0 mL of KOH 125.0 mL of KOHarrow_forwardAn analytical chemist is titrating 149.7 mL of a 0.5500M solution of benzoic acid (HC H CO,) with a 0.8000 M solution of NaOH. The p K of benzoic acid is 4.20. Calculate the pH of the acid solution after the chemist has added 16.29 mL of the NaOH solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of NaOH solution added. Round your answer to 2 decimal places. do pH = Check Explanation 2021 McGraw-Hil Education. All Rights Reserved. Terms of Use Privacy Accessibility V I 11:55 acer &arrow_forwardA chemistry graduate student is given 100. mL of a 0.40M nitrous acid (HNO,) solution. Nitrous acid is a weak acid with K 4.5 x 10. What mass of -4 NaNO, should the student dissolve in the HNO, solution to turn it into a buffer with pH =3.16? %3D You may assume that the volume of the solution doesn't change when the NaNO, is dissolved in it. Be sure your answer has a unit symbol, and round it to 2 significant digits. x10 Submit Assignment Continue 2021 McGraw Hill LLC. AlI Rights Reserved. Terms of Use Privacy Center | Accessibility MacBook Air F11 Figarrow_forward
- An analytical chemist is titrating 152.0 mL of a 0.2100 M solution of benzoic acid (HCH,CO,) with a 0.6700 M solution of NaOH. The p K, of benzoic acid is 4.20. Calculate the pH of the acid solution after the chemist has added 29.70 mL of the NaOH solution to it.arrow_forwardAn analytical chemist is titrating 64.9 mL of a 0.5800M solution of isopropylamine ((CH3),CHNH2) with a 0.3400M solution of HNO3. The p K, of isopropylamine is 3.33. Calculate the pH of the base solution after the chemist has added 69.8 mL of the HNO, solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HNO,3 solution added. Round your answer to 2 decimal places. pH =arrow_forwardAn analytical chemist is titrating 225.8 mL of a 0.5300 M solution of butanoic acid (HC,H,CO,) with a 0.8200 M solution of KOH. The p K, of butanoic acid is 4.82. Calculate the pH of the acid solution after the chemist has added 40.67 mL of the KOH solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of KOH solution added. Round your answer to 2 decimal places. pH = ||arrow_forward
- An analytical chemist is titrating 111.5 mL of a 0.2600M solution of acetic acid (HCH,CO,) with a 0.5100M solution of NaOH. The p K, of acetic acid is 4.70. Calculate the pH of the acid solution after the chemist has added 10.45 mL of the NaOH solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of NaOH solution added. Round your answer to 2 decimal places. pH = |arrow_forwardDetermine the mass of solid NaCH₃COO that must be dissolved in an existing 500.0 mL solution of 0.200 M CH₃COOH to form a buffer with a pH equal to 5.00. The value of Ka for CH₃COOH is 1.8 × 10⁻⁵. Based on the information provided, set up a RICE/ICE Table to answer the question. Let x represent the original concentration of CH₃COOH in the water.arrow_forwardin an acidbase titration, 10.0 mL (VB)of NH3 (Ka(NH+) = 5.8*10^ - 19) 0.18 M (CB) are titrated at the equivalence point (EP) with 10.0 ml (VA) of HCI 0.18 M (CA). Calculate (to two decimal places) the pH of the solution at the equivalence point.arrow_forward
- A chemist titrates 100.0 mL of a 0.5181 M carbonic acid (H,CO, solution with 0.7276 M NaOH solution at 25 °C. Calculate the pH at equivalence. The p K, of carbonic acid is 3.60. Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of NaOH solution added.arrow_forwardCalculating the pH at equivalence of a titration A chemist titrates 170.0 mL of a 0.2614M cyanic acid (HCNO) solution with 0.7980M NaOH solution at 25 °C. Calculate the pH at equivalence. The p K, of cyanic acid is 3.46. Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of NAOH solution added. pH =arrow_forwardIn this lab, we will experimentally determine the titration curve for the titration of a weak acid solution against a strong base solution. The weak acid we will use is potassium hydrogen phthalate, KHP, its chemical structure is shown below. KHP is an ionic compound composed of a potassium cation K+ and a hydrogen phthalate anion HP–. HP– is a weak acid and upon dissolving in water, can lower the pH of the solution. (a) Suggest the chemical reaction(s) when a solid sample of KHP is dissolved in water, writing out the chemical equations for them. (b) Sketch the structure of KHP from above and circle the hydrogen atom that is responsible for its acidity. (c) Calculate the pH of a solution made of 0.50 g of KHP and 50 mL of water. KHP has a molar mass of 204.2 g and at 25 °C has a pKa of 5.4.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY