
Chemistry: The Molecular Science
5th Edition
ISBN: 9781285199047
Author: John W. Moore, Conrad L. Stanitski
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
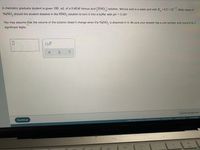
Transcribed Image Text:A chemistry graduate student is given 100. mL of a 0.40M nitrous acid (HNO,) solution. Nitrous acid is a weak acid with K 4.5 x 10. What mass of
-4
NaNO, should the student dissolve in the HNO, solution to turn it into a buffer with pH =3.16?
%3D
You may assume that the volume of the solution doesn't change when the NaNO, is dissolved in it. Be sure your answer has a unit symbol, and round it to 2
significant digits.
x10
Submit Assignment
Continue
2021 McGraw Hill LLC. AlI Rights Reserved. Terms of Use Privacy Center | Accessibility
MacBook Air
F11
Fig
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A buffer solution is prepared by adding 5.50 g of ammonium chloride and 0.0188 mol of ammonia to enough water to make 155 mL of solution. (a) What is the pH of the buffer? (b) If enough water is added to double the volume, what is the pH of the solution?arrow_forwardSketch the titration curve for a weak acid titrated by a strong base. When performing calculations concerning weak acidstrong base titrations, the general two-slep procedure is to solve a stoichiometry problem first, then to solve an equilibrium problem to determine the pH. What reaction takes place in the stoichiometry part of the problem? What is assumed about this reaction? At the various points in your titration curve, list the major species present after the strong base (NaOH, for example) reacts to completion with the weak acid, HA. What equilibrium problem would you solve at the various points in your titration curve to calculate the pH? Why is pH 7.0 at the equivalence point of a weak acid-strong base titration? Does the pH at the halfway point to equivalence have to be less than 7.0? What does the pH at the halfway point equal? Compare and contrast the titration curves for a strong acidstrong base titration and a weak acidstrong base titration.arrow_forwardA 1.0-L solution that is 4.2 M in ammonia is mixed with 26.7 g of ammonium chloride. a What is the hydroxide-ion concentration of this solution? b 0.075 mol of MgCl2 is added to the above solution. Assume that there is no volume change. After Mg(OH)2 has precipitated, what is the molar concentration of magnesium ion? What percent of the Mg2+ is removed from solution?arrow_forward
- A solution contains 0.00740 M calcium ion. A concentrated sodium fluoride solution is added dropwise to precipitate calcium fluoride (assume no volume change). a At what concentration of F does precipitate start to form? b When [F] = 9.5 104 M, what is the calcium-ion concentration? What percentage of the calcium ion has precipitated?arrow_forwardA buffer is prepared using the butyric acid/butyrate (HC4H7O2/C4H7O2)acid-base pair. The ratio of acid to base is 2.2 and Ka for butyric acid is1.54105. (a) What is the pH of this buffer? (b) Enough strong base is added to convert 15% of butyric acid to the butyrate ion. What is the pH of the resulting solution? (c) Strong acid is added to the buffer to increase its pH. What must the acid/base ratio be so that the pH increases by exactly one unit (e.g., from 2 to 3) from the answer in (a)?arrow_forwardAn aqueous solution of 0.057 M weak acid, HX, has a pH of 4.65. What is the pH of the solution if 0.018 mol of KX is dissolved in one liter of the weak acid?arrow_forward
- A saturated solution of a slightly soluble electrolyte in contact with some of the solid electrolyte is said to be a system in equilibrium. Explain. Why is such a system called a heterogeneous equilibrium?arrow_forwardConsider the nanoscale-level representations for Question 110 of the titration of the aqueous weak acid HX with aqueous NaOH, the titrant. Water molecules and Na+ ions are omitted for clarity. Which diagram corresponds to the situation: After a very small volume of titrant has been added to the initial HX solution? When enough titrant has been added to take the solution just past the equivalence point? Halfway to the equivalence point? At the equivalence point? Nanoscale representations for Question 110.arrow_forwardThe three flasks shown below depict the titration of an aqueous NaOH solution with HCl at different points. One represents the titration prior to the equivalence point, another represents the titration at the equivalence point, and the other represents the titration past the equivalence point. (Sodium ions and solvent water molecules have been omitted for clarity.) a Write the balanced chemical equation for the titration. b Label each of the beakers shown to indicate which point in the titration they represent. c For each solution, indicate whether you expect it to be acidic, basic, or neutral.arrow_forward
- Which of the following will affect the total amount of solute that can dissolve in a given amount of solvent? a. The solution is stirred. b. The solute is ground to fine particles before dissolving. c. The temperature changes.arrow_forwardConsider the titration of HF (K a=6.7104) with NaOH. What is the pH when a third of the acid has been neutralized?arrow_forwardA monoprotic organic acid that has a molar mass of 176.1 g/mol is synthesized. Unfortunately, the acid produced is not completely pure. In addition, it is not soluble in water. A chemist weighs a 1.8451-g sample of the impure acid and adds it to 100.0 mL of 0.1050 M NaOH. The acid is soluble in the NaOH solution and reacts to consume most of the NaOH. The amount of excess NaOH is determined by titration: It takes 3.28 mL of 0.0970 M HCl to neutralize the excess NaOH. What is the purity of the original acid, in percent?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning